ABSTRACT
- Two Gram-stain-positive, facultatively anaerobic, rod-shaped, and non-motile lactic acid bacterial strains, designated as strains CBA3605T and CBA3606T, were isolated from kimchi, a traditional Korean fermented food. Both strains were oxidase- and catalase-negative, non-spore-forming, non-hemolytic, and non-gas-producing. Optimal growth conditions for the two strains were observed at 30°C, pH 5.0, and 0% NaCl. The two genomes were composed of a circular chromosome and three plasmids and the DNA G + C content of 43.0%, respectively. Strains CBA3605T and CBA3606T were most closely related to Lactiplantibacillus (Lp.) pingfangensis 382-1T with 16S rRNA sequence similarity of 99.4% and 99.1%, respectively. However, the orthologous average nucleotide identities between CBA3605T and CBA3606T were 91.7%, and those with strain 382-1T were 76.9% and 76.5%, respectively. Digital DNA–DNA hybridization values between CBA3605T and CBA3606T were 45.0%, and those with strain 382-1T were 21.4% and 21.0%, respectively. The major fatty acids detected in both strains included C16:0, C18:1 ω9c, and summed features 7 (C19:1 ω7c, C19:1 ω6c, C19:0 cyclo ω10c, and/or C19:0 ω6c). The peptidoglycan of both strains CBA3605T and CBA3606T contained meso-diaminopimelic acid and was classified as A4α type (L-Lys–D-Asp). In polar lipid analyses, only strain CBA3605T contained aminophosphoglycolipid, which was absent in CBA3606T, although both strains harbored same major polar lipids (diphosphatidylglycerol, phosphatidylglycerol, and phosphatidylethanolamine). Based on phenotypic, phylogenetic, genomic, biochemical, and chemotaxonomic analyses, strains CBA3605T and CBA3606T represent two novel species of the genus Lactiplantibacillus, for which the names Lactiplantibacillus koreensis sp. nov. and Lactiplantibacillus kimchii sp. nov. are proposed, with CBA3605T (= KACC 81073BPT = JCM 37965T), and CBA3606T (= KACC 81074BPT = JCM 37966T) as the type strains.
-
Keywords: Lactiplantibacillus koreensis, Lactiplantibacillus kimchi, strain CBA3605T, strain CBA3606T, kimchi, lactic acid bacteria
Introduction
In 2020, the genus Lactobacillus (Lb.) was reclassified into 25 distinct genera based on a polyphasic taxonomic approach (Zheng et al., 2020). One of the newly established genera is Lactiplantibacillus (Lp.), characterized as facultatively anaerobic, homofermentative, Gram-stain-positive, rod-shaped, non-motile, non-spore-forming, and urease-negative. They are able to ferment a wide range of carbohydrates. Most species metabolize phenolic acids through esterase, decarboxylase, and reductase activities, while some exhibit pseudocatalase activity or reduce nitrate under specific conditions. Lactiplantibacillus species possess relatively large genomes, with sizes generally between 2.63 and 3.65 Mbp, and their DNA G + C contents range from 42.9 to 48.7 mol%. The name Lactiplantibacillus is derived from Latin: lactis (milk), planta (plant), and bacillus (rod), reflecting its origin as a group of rod-shaped lactic acid bacteria (LAB) associated with plants. In line with its name, most species of Lactiplantibacillus have been initially isolated from fermented or fermenting plant-based foods, such as Ghanaian fermenting cocoa beans (De Bruyne et al., 2009), Chinese fermented radish pickles (Mao et al., 2015), Chinese fermented cabbage pickles (Liu and Gu, 2019), and Russian sauerkraut (Heng et al., 2023). Lp. plantarum DSM 20174T, the type species of Lactiplantibacillus, was also isolated from pickled cabbages (Orla-Jensen, 1919). However, no novel species of this genus has been reported from kimchi, which is one of the most well-known plant-based fermented foods.
In this study, we report two novel strains, CBA3605T and CBA3606T, isolated from kimchi and belonging to the genus Lactiplantibacillus. Based on a polyphasic taxonomic approach, including phylogenetic, genomic, physiological, biochemical, and chemotaxonomic analyses, we propose that strains CBA3605T and CBA3606T represent two novel Lactiplantibacillus species.
Materials and Methods
Isolation and culture conditions
Strains CBA3605T and CBA3606T were isolated from Korean cabbage kimchi in Gwangju, Republic of Korea. Kimchi soup samples were filtered using sterile stomacher filter bags (Nasco) and then serially diluted in 0.85% (w/v) saline solution (3MTM). The diluted samples were spread onto de Man, Rogosa, and Sharpe (MRS; BD Difco) agar (Lee et al., 2020) and incubated at 30°C for 2 days. Single colonies were streaked at least three times onto fresh MRS agar to ensure purity. The identified strains, CBA3605T and CBA3606T, were preserved at –80°C in 10% (v/v) skim milk for further experiments. The two strains were deposited in the Korean Agricultural Culture Collection (accession numbers KACC 81073BPT and 81074BPT) and the Japan Collection of Microorganisms (JCM; accession numbers JCM 37965T and 37966T). For taxonomic comparison, phylogenetically related type strains were obtained from the National Collection of Industrial Food and Marine Bacteria (NCIMB) and the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ): Lp. daoliensis NCIMB 15181T, Lp. nangangensis NCIMB 15186T, Lp. pingfangensis NCIMB 15187T, and Lp. brownii DSM 116485T. All six strains were cultured under identical conditions for genomic, physiological, biochemical, and chemotaxonomic characterization. To compare their characteristics with Lp. plantarum DSM 20174T, the type species of Lactiplantibacillus, publicly available data from a previous study were used (Curk et al., 1996).
16S rRNA gene-based phylogenetic analysis
The 16S rRNA gene sequences of strains CBA3605T and CBA3606T were amplified using PCR pre-mix (Bioneer) and the universal bacterial primers 27F (5'-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′-TAC GGY TAC CTT GTT ACG ACT T-3′) (Lane, 1991). The PCR products were purified and subsequently sequenced to obtain high-quality sequences using a combination of universal bacterial primers, including 27F, 519F, 800R, and 1492R. The 16S rRNA gene sequence similarities between the two strains and validly published type strains were determined using the EzBioCloud server (Yoon et al., 2017). The sequences were aligned using the ClustalW algorithm implemented in SeqMan Pro (DNASTAR) (Burland, 1999) without considering secondary structure, and the phylogenetic trees were constructed using MEGA11 software based on the neighbor-joining (NJ), maximum likelihood (ML), and maximum parsimony (MP) algorithms (Tamura et al., 2021). Bootstrap values were calculated from 1,000 replications. Evolutionary distances were computed using the Kimura two-parameter method (Felsenstein, 1985). Levilactobacillus tangyuanensis 137-3T (GenBank accession number MK110859) was used as the outgroup in the phylogenetic trees.
Genome-based analysis
Whole-genome sequencing of strains CBA3605T and CBA3606T was performed at Macrogen (Republic of Korea) using combined sequencing technologies. Long-read and short-read sequencing were conducted using the PacBio Sequel IIe platform with a 15 kb SMRTbellTM template library and the Illumina HiSeq X Ten platform, respectively. PacBio reads were de novo assembled using the Microbial Assembly application in SMRT Link v11.0, and the assemblies were polished with Pilon v1.21 using the Illumina reads to correct base errors, misassemblies, and fill gaps. Annotation of the complete genome sequences was performed using the NCBI Prokaryotic Genome Automatic Annotation Pipeline (PGAP) (Tatusova et al., 2016). The DNA G + C content of strains CBA3605T and CBA3606T was calculated from their assembled genomes. The completeness and contamination metrics were assessed using CheckM v1.2.3 (Parks et al., 2015). Functional annotation of genes was conducted using the standalone eggNOG-mapper v2 (Cantalapiedra et al., 2021) with default settings, based on the Clusters of Orthologous Groups (COG) database. The proportion of genes assigned to each COG category was calculated as a percentage. To investigate the genomic differences between strains CBA3605T and CBA3606T, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was performed. The Blast KEGG Orthology And Links Annotation (BlastKOALA) was used for the functional annotation of the predicted proteins in both strains (Kanehisa et al., 2016). Orthologous average nucleotide identity (OrthoANI) and digital DNA–DNA hybridization (dDDH) values were calculated from the whole-genome sequences using the Orthologous ANI Tool (OAT; https://www.ezbiocloud.net/tools/orthoani) (Lee et al., 2016) and the Genome-to-Genome Distance Calculator version 3.0 (GGDC 3.0; https://ggdc.dsmz.de/ggdc.php) (Goris et al., 2007), respectively. To construct a phylogenomic tree, the genome sequences of strains CBA3605T, CBA3606T, and closely related Lactiplantibacillus species with publicly available genomes were retrieved from NCBI GenBank (https://www.ncbi.nlm.nih.gov/genome/). The genome sequence of Lp. plantarum DSM 20174T was retrieved and included in the comparative analysis. A phylogenomic tree was reconstructed using the up-to-date bacterial core gene (UBCG2) pipeline (Kim et al., 2021), which employs a set of 100 conserved bacterial core genes. The core genes were extracted from the genomes of interest, individually aligned, and concatenated to construct a maximum-likelihood (ML) phylogenetic tree implemented in FastTree (Price et al., 2009).
Physiological, biochemical, and chemotaxonomic analyses
Strains CBA3605T, CBA3606T, and four closely related type strains were pre-cultured on MRS agar at 30°C for 2 days for physiological, biochemical, and chemotaxonomic analyses. All six strains were subsequently subjected to experiments, conducted at 30°C for 2 days unless otherwise specified. Optimal growth conditions for pH, temperature, and NaCl concentration were assessed using MRS medium. The pH tolerance was tested in MRS broth adjusted to pH 3.0–9.0 at 1.0 unit intervals and incubated for 2 days. MRS broths at pH 3.0–6.0, pH 6.0–8.0, and pH 8.0–9.0 were prepared using citric acid/sodium citrate, Na2HPO4/NaH2PO4 and Tris–HCl buffers (Gomori, 2010), respectively. The pH levels were readjusted after autoclaving at 121°C for 15 min. Temperature tolerance was tested on MRS agar at 5–50°C in 5°C intervals, and NaCl tolerance was tested at 0–10% NaCl in 2% intervals. Anaerobic growth was examined on MRS agar under anaerobic conditions using a BBL GasPak Plus system (BD Biosciences) for 2 days at 30°C (Agarwal et al., 2006). Motility was assessed in MRS broth containing 0.2% agar for 2 days at 30°C (Cappuccino and Sherman, 2008) and spore formation was evaluated by exposing the strains to 100°C for 30 min using a heating block, followed by incubation on MRS agar at 30°C for 2 days to assess the growth of heat-resistant spores (Kent et al., 2016). Gas production from glucose was tested in MRS broth using Durham tubes. Gram staining was performed using a Gram stain kit (Becton, Dickinson and Company) according to the manufacturer’s instructions. Cell morphology of strains CBA3605T and CBA3606T was observed using a scanning electron microscope (SEM, Verios 5 XHR, Thermo Fisher Scientific). For the sample preparation, cells were washed three times with sterile distilled water, dropped onto silicon wafers, and air-dried for 3 days. The dried samples were coated with a 20 nm platinum layer and observed by SEM. Biochemical features, including carbon source utilization, were analyzed using the API 50 CHL (bioMérieux) system, following the manufacturer’s instructions. Catalase test was conducted using an oxygen bubble formation in a 3% (v/v) hydrogen peroxide solution (Whittenbury, 1964). Oxidase activity was determined based on color changes observed on oxidase test discs. Hemolytic activity was evaluated on MRS agar supplemented with 5% (v/v) sheep blood. Tellurite tolerance was tested using MRS agar supplemented with 0.04% (w/v) potassium tellurite (K2TeO3, Sigma-Aldrich). The isomeric composition of lactic acid was determined enzymatically using the D/L-lactate test kit (Megazyme). Cellular fatty acids were extracted from strains at the same growth phase and analyzed following the protocol of the Sherlock Microbial Identification System (MIDI) (Sasser, 1990). Fatty acid methyl esters were separated by gas chromatography (model 6890, Hewlett Packard), and identified using the MOORE6 database in the Sherlock software version 6.0B (Miller and Berger, 1985). Peptidoglycan amino acids were extracted and hydrolyzed in 6 N HCl at 121°C for 15 min. The hydrolysates were subjected to thin-layer chromatography (TLC) as described previously (Schleifer and Kandler, 1972), and amino acid components were visualized with ninhydrin. Polar lipids were obtained from cells harvested during the exponential growth phase and analyzed by TLC according to standard procedures (Minnikin et al., 1977). Specific lipid classes were detected using following reagents: 10% ethanolic molybdophosphoric acid for total polar lipids, ninhydrin for aminolipids, the Dittmer-Lester reagent for phospholipids, and α-naphthol/sulfuric acid for glycolipids.
Results and Discussion
16S rRNA gene-based phylogeny
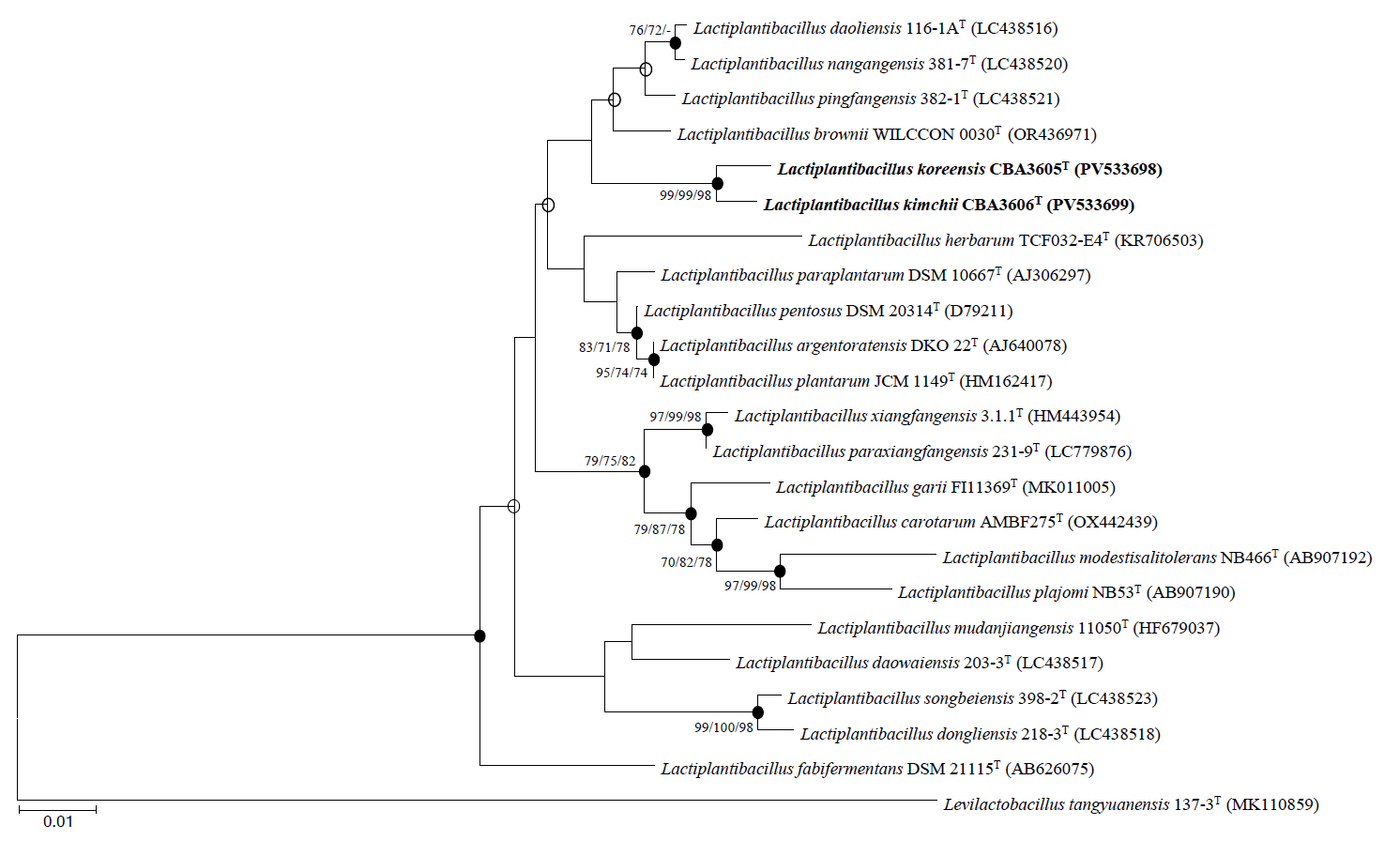
Phylogenetic trees based on 16S rRNA gene sequences were constructed using the NJ (Fig. 1), MP (Fig. S1A), and ML (Fig. S1B) algorithms. All trees consistently indicated that strains CBA3605T and CBA3606T belong to the genus Lactiplantibacillus and are most closely related to Lp. daoliensis 116-1AT, Lp. nangangensis 381-7T, Lp. pingfangensis 382-1T, and Lp. brownii WILCCON 0030T. Their 16S rRNA gene sequence similarities with strain CBA3605T were 99.1%, 99.0%, 99.4%, and 99.1%, while those with strain CBA3606T were 98.8%, 98.7%, 99.1%, and 98.9%, respectively. Interestingly, similar to strains CBA3605T and CBA3606T, which were isolated from kimchi, a traditional Korean fermented food, the four closely related type strains were also isolated from fermented foods such as pickles and sauerkraut (Heng et al., 2023; Liu and Gu, 2019). The similarity between strains CBA3605T and CBA3606T was 99.8%. Based on these phylogenetic relationships, further analyses, including genomic, physiological, biochemical, and chemotaxonomic characterization, were conducted using all six strains.
Genomic analysis
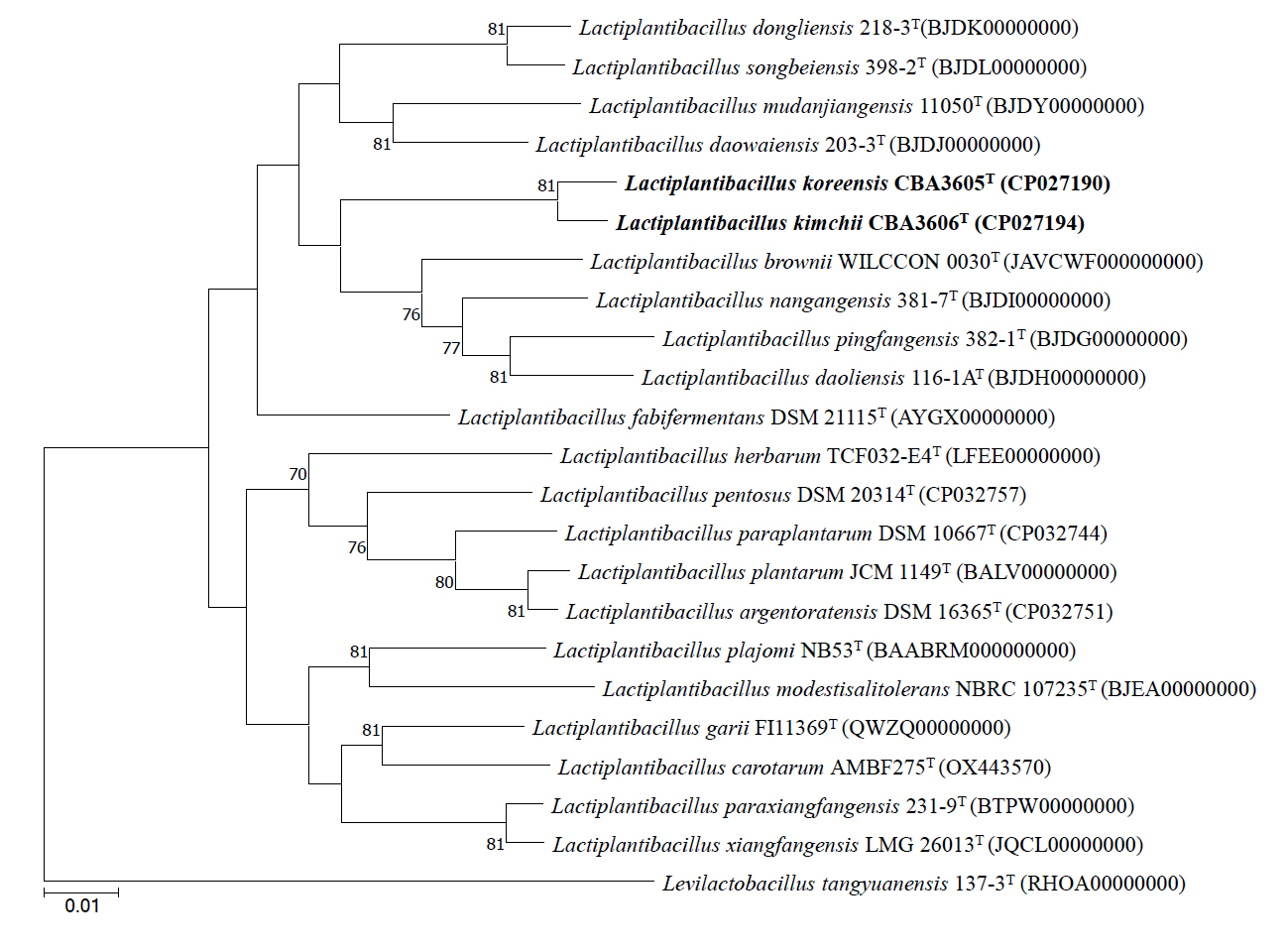
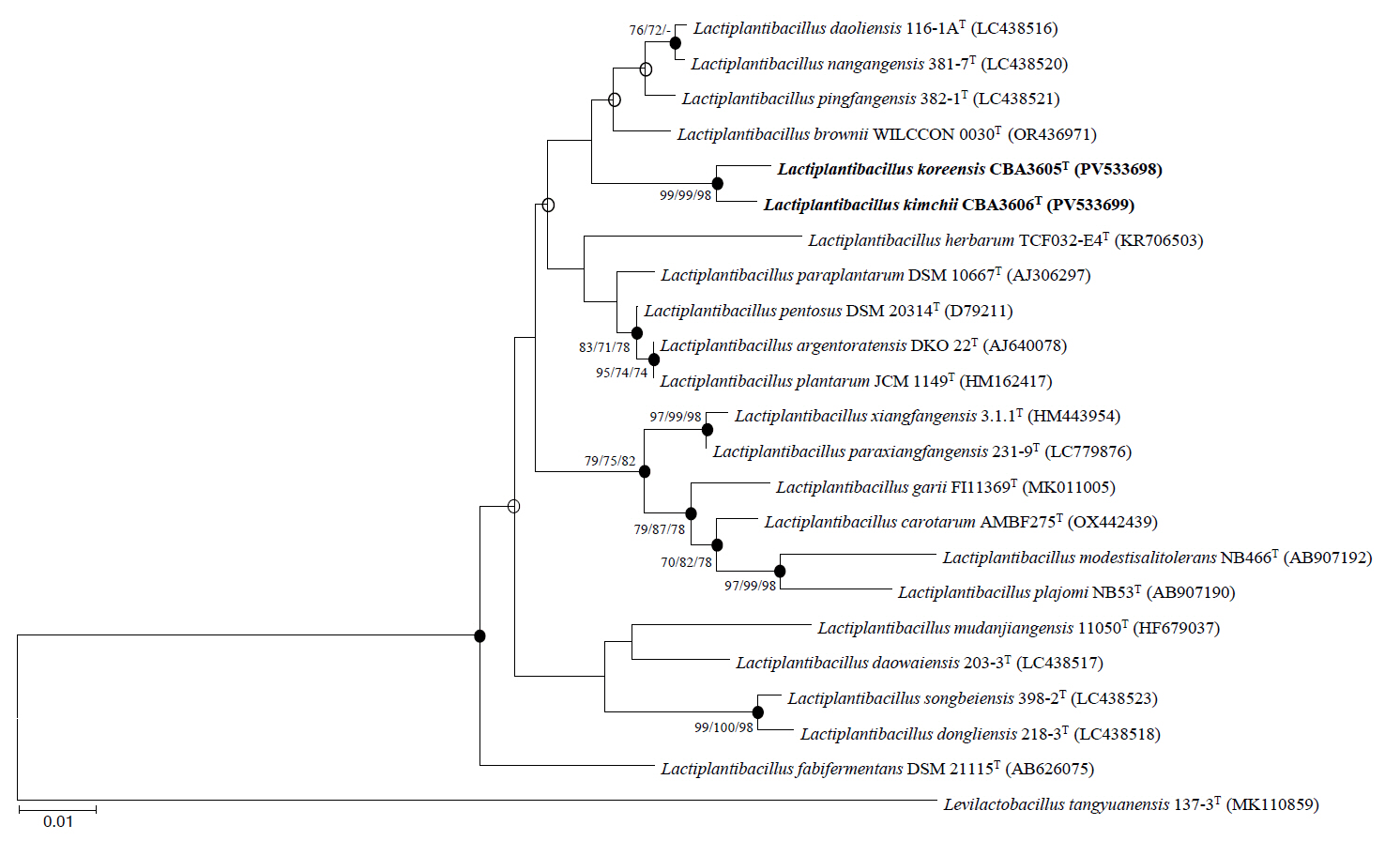
Consistent with the 16S rRNA gene-based phylogeny, whole genome phylogenetic analysis revealed that strains CBA3605T and CBA3606T are most closely related to Lp. daoliensis 116-1AT, Lp. nangangensis 381-7T, Lp. pingfangensis 382-1T, and Lp. brownii WILCCON 0030T, while forming a distinct clade separate from the four Lactiplantibacillus type strains (Fig. 2). The general genomic features of these two strains, their closely related type strains, and Lp. plantarum DSM 20174T (type species) are summarized in Table 1. Notably, the genome sizes of strains CBA3605T and CBA3606T were approximately 2.4–2.5 Mbp, which are smaller than those of other species that range from 2.6 to 3.3 Mbp. Strains CBA3605T and CBA3606T also harbored lower total gene and protein-coding gene counts compared to Lp. nangangensis 381-7T, Lp. pingfangensis 382-1T, Lp. brownii WILCCON 0030T, and Lp. plantarum DSM 20174T. However, the numbers of 5S rRNA, 16S rRNA, 23S rRNA, and tRNA genes in strains CBA3605T and CBA3606T were higher than those of Lp. daoliensis 116-1AT, Lp. nangangensis 381-7T, and Lp. pingfangensis 382-1T. Although the two strains exhibited different genomic features compared to their closest species, appropriate thresholds are required to determine their distinction. Moreover, such thresholds are necessary to evaluate whether strains CBA3605T and CBA3606T themselves represent different species. To clarify this point, OrthoANI and dDDH analyses were performed (Table 2). OrthoANI and dDDH values between the two strains were 91.7% and 45.0%, respectively, which are below the commonly accepted species thresholds of 95–96% for OrthoANI (Kim et al., 2014) and 70% for dDDH (Goris et al., 2007), indicating that the strains represent distinct species. Additionally, both strains showed considerably low OrthoANI and dDDH values when compared with the type strains of Lactiplantibacillus, further supporting their novelty.
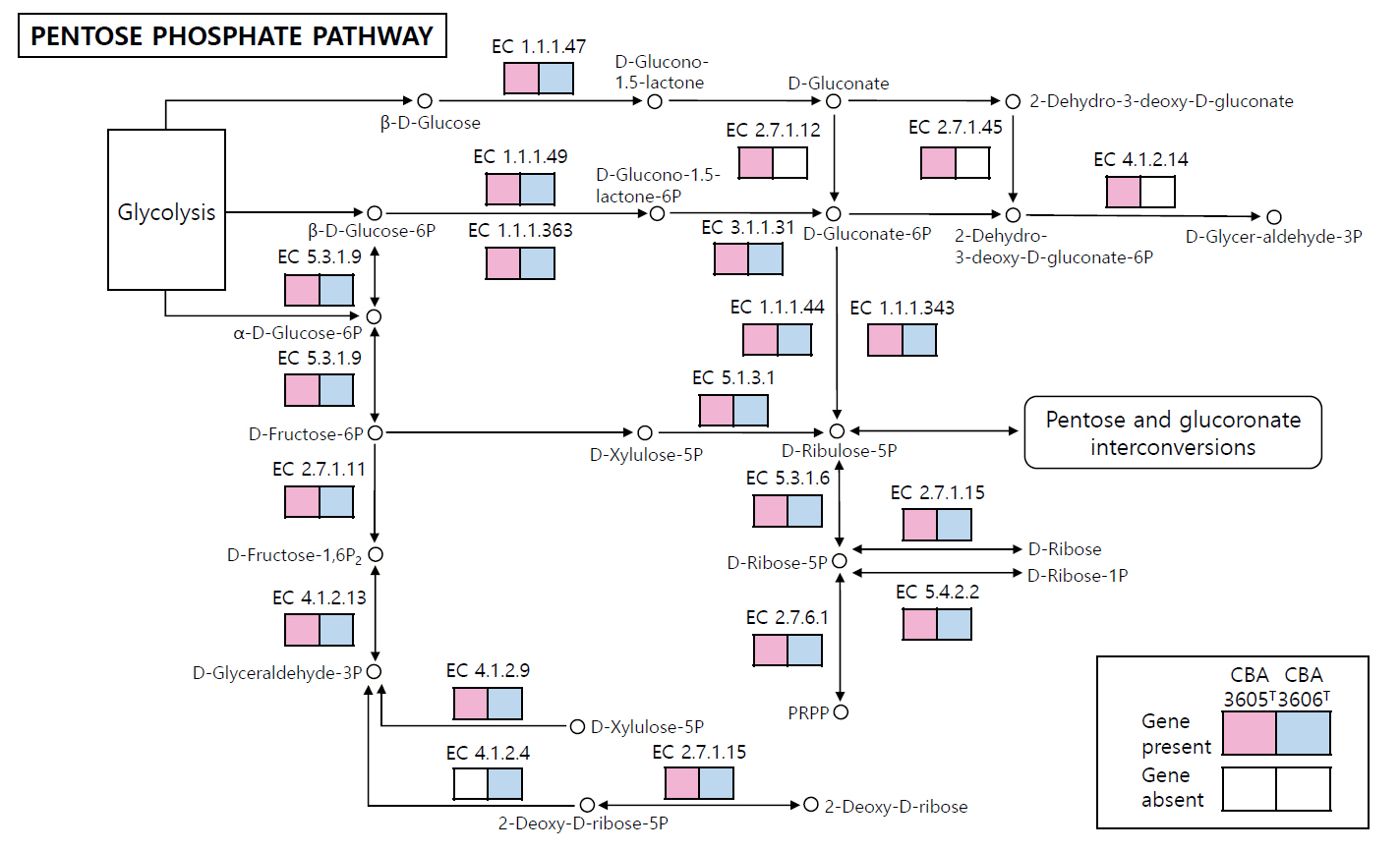
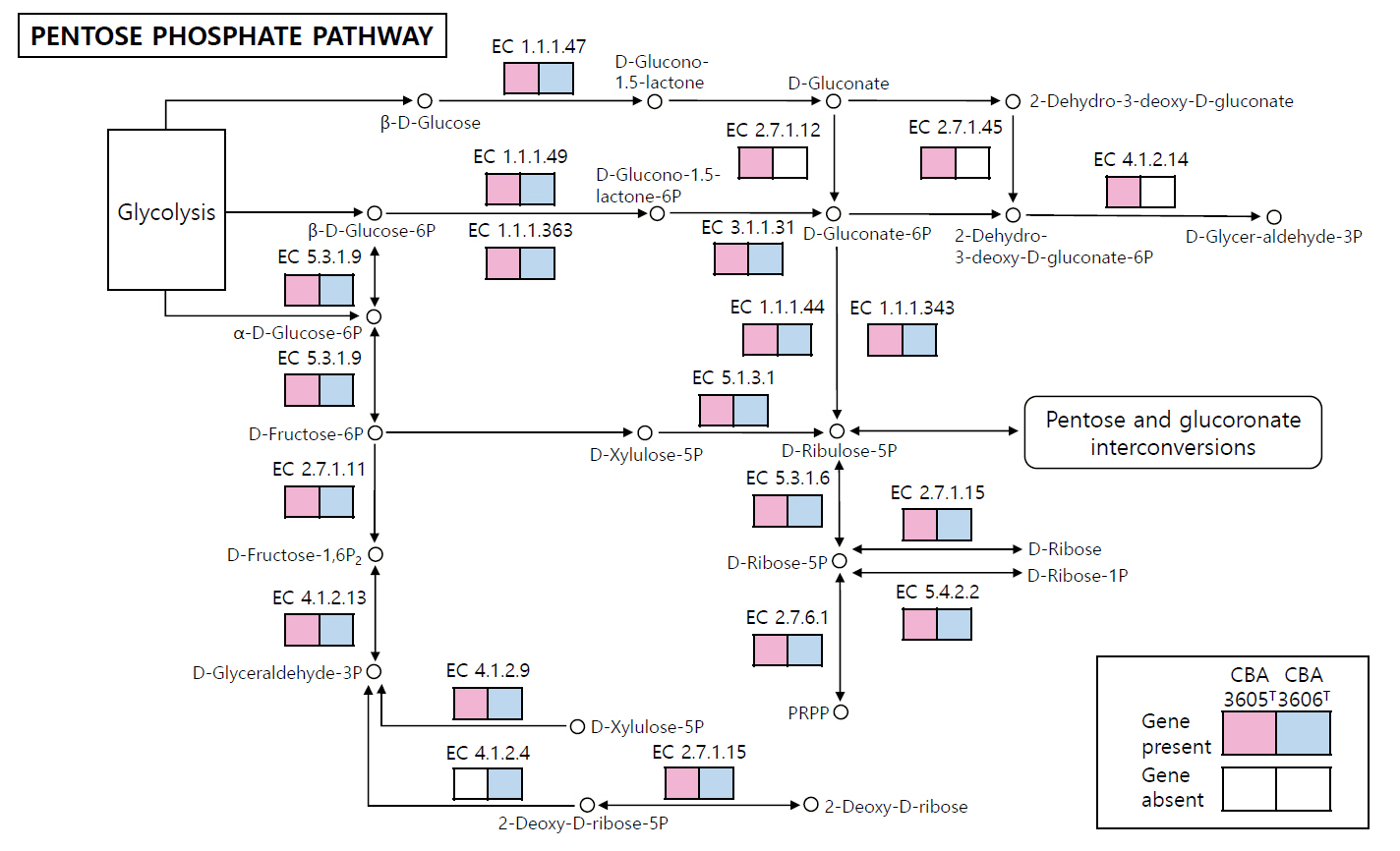
The COG category distributions of strains CBA3605T, CBA3606T, Lp. daoliensis 116-1AT, Lp. nangangensis 381-7T, Lp. pingfangensis 382-1T, Lp. brownii WILCCON 0030T, and Lp. plantarum DSM 20174T are summarized in Table S1. In all seven strains, the largest proportion of genes was assigned to category K (transcription), ranging from 10.1% to 12.3%. In strains CBA3605T and CBA3606T, the second most abundant categories were J (translation, ribosomal structure, and biogenesis) and L (DNA replication, recombination, and repair), respectively, while the third most enriched categories were G (carbohydrate transport and metabolism) and J, respectively. This suggests functional divergence between the two strains. We further investigated the distinct genomic features between strains CBA3605T and CBA3606T using KEGG analysis (Fig. 3). In the metabolic pathways, especially in the pentose phosphate pathway, strain CBA3605T harbored the genes encoding gluconokinase (EC 2.7.1.12), 2-dehydro-3-deoxygluconokinase (EC 2.7.1.45), and 2-dehydro-3-deoxyphosphogluconate aldolase (EC 4.1.2.14), which were absent in strain CBA3606T. In contrast, the gene encoding deoxyribose-phosphate aldolase (EC 4.1.2.4) was present in strain CBA3606T but absent in strain CBA3605T. These findings support the differentiation between strains CBA3605T and CBA3606T.
In addition to these genomic differences, KEGG analysis suggested that both strains are homofermentative. They harbored the gene encoding fructose-bisphosphate aldolase (EC 4.1.2.13; locus_tags C5Z25_RS08140 in CBA3605 and C5Z26_RS09410 in CBA3606), a key enzyme present in homofermentative but absent in heterofermentative LAB strains (Chun et al., 2017). To further confirm this, we reconstructed the metabolic pathways from glucose to lactate using strains CBA3605T and CBA3606T, together with Lp. plantarum DSM 20174T (homofermentative control) and Leuconostoc (Leu.) mesenteroides ATCC 8293T (heterofermentative control) (Fig. S2). The reconstructed pathway clearly showed that strains CBA3605T and CBA3606T harbored the complete set of glycolytic genes, including phosphofructokinase-1 (EC 2.7.1.11) and fructose-biphosphate aldolase (EC 4.1.2.13), consistent with homofermentative metabolism. In contrast, the heterofermentative strain Leu. mesenteroides ATCC 8293T lacked these key genes, relying instead on the phosphoketolase pathway (Wang et al., 2021). These results demonstrate that strains CBA3605T and CBA3606T are homofermentative members of the genus Lactiplantibacillus.
Lastly, considering that the genomes of strains CBA3605T and CBA3606T were complete sequences, we further analyzed their plasmid features using PGAP (Table S2). Both strains possessed three plasmids, but the total plasmid sizes and gene numbers in strain CBA3605T were smaller than those in strain CBA3606T. Although most plasmid-borne genes were shared between the two strains, comparative analysis of plasmid sequences revealed 12 unique genes in CBA3605T and 28 unique genes in CBA3606T, respectively (Table S3).
Taken together, the 16S rRNA gene sequence and whole-genome analyses suggest that strains CBA3605T and CBA3606T represent novel species within the genus Lactiplantibacillus. Further physiological, biochemical, and chemotaxonomic analyses were carried out to support this taxonomic classification.
Physiological, biochemical, and chemotaxonomic analyses
Tables 3 and 4 presents the phenotypic characteristics of strains CBA3605T and CBA3606T, and their closely related type strains. All six strains shared identical growth conditions at 30°C and 0% (w/v) NaCl, whereas their optimal pH values differed. Strains CBA3605T, CBA3606T, Lp. nangangensis NCIMB 15186T, and Lp. pingfangensis NCIMB 15187T exhibited optimal growth at pH 5, while Lp. daoliensis NCIMB 15181T and Lp. brownii DSM 116485T exhibited at pH 6 and 7, respectively. All strains formed beige-colored colonies, except Lp. brownii DSM 116485T, which formed white colonies. Other physiological characteristics were also consistent among the six strains as they were facultatively anaerobic, non-motile, non-spore-forming, oxidase- and catalase-negative, Gram-positive, non-gas-producing, non-hemolytic, and not resistant to tellurite. However, the two strains and their closely related type strains exhibited different acid production patterns. Strains CBA3605T and CBA3606T produced acid from D-ribose, L-rhamnose, D-sorbitol, and gentiobiose, but not from D-raffinose and D-turanose. In contrast, the remaining closely related type strains did not produce acid from L-rhamnose, D-sorbitol. Strain CBA3605T produced acid from D-lactose, but not from methyl-α-D-glucopyranoside, N-Acetylglucosamine while strain CBA3606ᵀ showed the opposite pattern. Notably, four closely related type strains produced acid from the latter two but not from D-lactose. The remaining close relatives exhibited the same acid production pattern as strain CBA3606T. Relevant data of Lp. plantarum DSM 20174T from a previous study (Curk et al., 1996) were also included in Table 3 for direct comparison of the six strains with the type species of Lactiplantibacillus. In contrast to the other six strains, Lp. plantarum DSM 20174T was able to grow in the presence of 8% NaCl but did not survive at pH 4. SEM images showed that strain CBA3605T was rod-shaped and measured approximately 1.8 ± 0.4 µm in length and 0.5 ± 0.1 µm in width. Strain CBA3606T also displayed a rod shaped, with a length of approximately 2.1 ± 0.8 µm and a width of 0.5 ± 0.2 µm (Fig. S3).
The predominant fatty acids of strains CBA3605T and CBA3606T, as well as the four closely related type strains, were C16:0, C18:1 ω9c, and Summed feature 7, which are typical for members of the genus Lactiplantibacillus. However, the relative proportions of these fatty acids varied among the strains. In particular, C18:1 ω9c was markedly higher in strains, 382-1T and WILCCON 0030T (39.4–40.2%) compared with strains CBA3605T, CBA3606T, 116-1AT, and Lp. nangangensis 381-7T (22.0–32.4%). Conversely, Summed feature 7 was most abundant in strains CBA3605T and CBA3606T (31.0–33.8%), but relatively lower in strains 382-1T and WILCCON 0030T (24.1–26.6%). C16:0 levels were relatively consistent (17.7–23.1%), although strains CBA3605T and CBA3606T showed slightly higher values than the others. These variations in fatty acid composition provide additional phenotypic evidence to differentiate the novel strains from their closest relatives.
TLC analysis of whole-cell hydrolysates showed a clear spot corresponding to meso-diaminopimelic acid (meso-DAP), which was confirmed by comparison with a standard (Fig. S4). Additional faint spots were observed at higher Rf values that are consistent with lysine and aspartic acid, which were further supported by genome annotation. Genome annotation supported these findings, revealing genes for meso-DAP biosynthesis, including asd (K00133; locus_tag=C5Z25_RS03555 in strain CBA3605T; locus_tag=C5Z26_RS04925 in strain CBA3606T), dapA (K01610; locus_tag=C5Z25_RS01900 in strain CBA3605T; locus_tag=C5Z26_RS03280 in strain CBA3606T), and dapF (K01778; locus_tag=C5Z25_RS02640 in strain CBA3605T; locus_tag=C5Z26_RS04020 in strain CBA3606T), as well as lysA (K01586; locus_tag=C5Z25_RS00380 in strain CBA3605T; locus_tag=C5Z26_RS02035 in strain CBA3606T) for conversion to L-Lys, and murM (K14188; locus_tag=C5Z25_RS01255 in strain CBA3605T; locus_tag=C5Z26_RS02905 in strain CBA3606T) and murN (K14189; locus_tag=C5Z25_RS05430 in strain CBA3605T; locus_tag=C5Z26_RS05305 in strain CBA3606T) for D-Asp cross-bridge formation. Taken together, the peptidoglycan of the strains is classified as A4α type (L-Lys–D-Asp).
Strains CBA3605T and CBA3606T shared the same major polar lipids, namely diphosphatidylglycerol (DPG), phosphatidylglycerol (PG), and phosphatidylethanolamine (PE), whereas distinct differences were observed in the minor components, with variations in both the number and intensity of phospholipid, aminolipid, and glycolipid spots on TLC plates. In particular, CBA3605T contained aminophosphoglycolipid (APGL), which was absent in CBA3606T (Fig. S5).
Taxonomic conclusion
The results of phylogenetic analyses based on 16S rRNA gene and genome sequences, as well as physiological and chemotaxonomic assessments, clearly demonstrate that strains CBA3605T and CBA3606T belong to the genus Lactiplantibacillus. The two strains have genome-based relatedness measures (ANI and dDDH) of 91.7% and 45.0%, respectively, which are well below the generally accepted species thresholds of 95–96% and 70%. Additionally, several distinct phenotypic characteristics suggest that strains CBA3605T and CBA3606T represent different novel species not recognized within the genus Lactiplantibacillus. In conclusion, based on the phylogenetic, physiological, and chemotaxonomic evidence, we propose the names Lactiplantibacillus koreensis sp. nov. for strains CBA3605T and Lactiplantibacillus kimchii sp. nov. for strains CBA3606T.
Description of Lactiplantibacillus koreensis sp. nov
Lactiplantibacillus koreensis (ko.re.en′sis. N.L. masc. adj. koreensis, pertaining to Korea, from where the type strain was isolated).
Cells are Gram-stain-positive, facultatively anaerobic, non-motile, non-hemolytic, and non-spore-forming. Cells are rod-shaped with approximately 1.8 ± 0.4 µm in length and 0.5 ± 0.1 µm in width. Colonies are beige-colored, convex, and smooth. Cells grow at 15–40°C (optimum at 30°C), pH 4.0–7.0 (optimum, pH 5.0), and 0–6.0% NaCl (optimum, 0%). Gas production from glucose, tellurite tolerance, and oxidase and catalase activities are negative. The strain produces D-lactic acid. In the API 50 CHL test, the strain exhibited positive reactions for D-ribose, D-galactose, D-glucose, D-fructose, D-mannose, L-rhamnose, D-mannitol, D-sorbitol, amygdalin, arbutin, esculin ferric citrate, salicin, D-cellobiose, D-maltose, D-lactose, D-sucrose, D-trehalose, gentiobiose, and potassium 2-ketogluconate. Negative reactions are observed for glycerol, erythritol, D-arabinose, L-arabinose, D-xylose, L-xylose, adonitol, methyl-β-D-xylopyranoside, L-sorbose, dulcitol, inositol, methyl-α-D-mannopyranoside, methyl-α-D-glucopyranoside, N-acetylglucosamine, D-melibiose, inulin, D-melezitose, D-raffinose, starch, glycogen, xylitol, D-turanose, D-lyxose, D-tagatose, D-fucose, L-fucose, D-arabitol, L-arabitol, potassium gluconate, and potassium 5-ketogluconate. Major cellular fatty acids are identified as C16:0, C18:1 ω9c, and summed features 7 (C19:1 ω7c, C19:1 ω6c, C19:0 cyclo ω10c, and/or C19:0 ω6c). The cell-wall peptidoglycan contains meso-diaminopimelic acid and is classified as A4α type (L-Lys–D-Asp). Major polar lipids consist of DPG, PG, and PE. Minor polar lipids include unidentified glycolipid 1 (GL1), unidentified glycolipid 2 (GL2), unidentified glycolipid 3 (GL3), unidentified glycolipid 4 (GL4), unidentified glycolipid 5 (GL5), unidentified glycolipid 6 (GL6), unidentified glycolipid 7 (GL7), unidentified lipid 1 (L1), unidentified lipid 2 (L2), phospholipid 1 (PL1), phospholipid 2 (PL2), phospholipid 3 (PL3), aminophospholipid 1 (APL1), aminophospholipid 2 (APL2), aminophospholipid 3 (APL3), and aminophosphoglycolipid (APGL).
The type strain, CBA3605T (=KACC 81073BPT = JCM 37965T), was isolated from cabbage kimchi in Gwangju, Republic of Korea. The whole-genome sequence of the type strain is 2,494,909 bp in length with a DNA G + C content of 43 mol%. GenBank accession numbers for the 16S rRNA gene and the genome sequence of the type strain are PV533698 and CP027190, respectively.
Description of Lactiplantibacillus kimchii sp. nov.
Lactiplantibacillus kimchii (kim′chi.i. M.L. gen. n. kimchii from kimchi, a Korean fermented vegetable food).
Cells are Gram-stain-positive, facultatively anaerobic, non-motile, non-hemolytic, and non-spore-forming. Cells are rod-shaped with approximately 2.1 ± 0.8 µm in length and 0.5 ± 0.2 µm in width. Colonies are beige-colored, flat, and dry. Cells grow at 15–40°C (optimum at 30°C), pH 4.0–7.0 (optimum, pH 5.0), and 0–6.0% NaCl (optimum, 0%). Gas production from glucose, tellurite tolerance, and oxidase and catalase activities are negative. The strain produces D-lactic acid. In API 50 CHL test, the strain exhibited positive reactions for D-ribose, D-galactose, D-glucose, D-fructose, D-mannose, L-rhamnose, D-mannitol, D-sorbitol, methyl-α-D-glucopyranoside, N-acetylglucosamine, amygdalin, arbutin, esculin ferric citrate, salicin, D-cellobiose, D-maltose, D-sucrose, D-trehalose, gentiobiose, and potassium 2-ketogluconate. Negative reactions are observed for glycerol, erythritol, D-arabinose, L-arabinose, D-xylose, L-xylose, adonitol, methyl-β-D-xylopyranoside, L-sorbose, dulcitol, inositol, methyl-α-D-mannopyranoside, D-lactose, D-melibiose, Inulin, D-melezitose, D-raffinose, starch, glycogen, xylitol, D-turanose, D-lyxose, D-tagatose, D-fucose, L-fucose, D-arabitol, L-arabitol, potassium gluconate, and potassium 5-ketogluconate. Major fatty acids are identified as C16:0, C18:1 ω9c, and summed features 7 (C19:1 ω7c, C19:1 ω6c, C19:0 cyclo ω10c, and/or C19:0 ω6c). The cell-wall peptidoglycan contains meso-diaminopimelic acid and is classified as A4α type (L-Lys–D-Asp). Major polar lipids consist of DPG, PG, and PE. Minor polar lipids include unidentified glycolipid 1 (GL1), unidentified glycolipid 2 (GL2), unidentified glycolipid 3 (GL3), unidentified glycolipid 4 (GL4), unidentified glycolipid 5 (GL5), unidentified glycolipid 6 (GL6), unidentified glycolipid 7 (GL7), unidentified lipid 1 (L1), unidentified lipid 2 (L2), phospholipid 1 (PL1), phospholipid 2 (PL2), phospholipid 3 (PL3), aminophospholipid 1 (APL1), aminophospholipid 2 (APL2), and aminophospholipid 3 (APL3).
The type strain, CBA3606T (=KACC 81074BPT = JCM 37966T), was isolated from cabbage kimchi in Gwangju, Republic of Korea. The whole-genome sequence of the type strain is 2,449,069 bp in length with a DNA G + C content of 43 mol%. GenBank accession numbers for the 16S rRNA gene and the genome sequence of the type strain are PV533699 and CP027194, respectively.
Acknowledgments
This work was supported by the World Institute of Kimchi (KE2501-1-1); the Agricultural Microbiome R&D Programs (RS-2024-00397218 and RS-2024-00401736) of the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET), funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA); and the Research Program for Agricultural Science and Technology Development (RS-2025-02263881) of the Rural Development Administration, Republic of Korea.
Conflict of Interest
The authors declare no competing financial conflicts of interest.
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.71150/jm.2507007.
Fig. S1.
Phylogenetic trees based on 16S rRNA gene sequences of strains CBA3605ᵀ, CBA3606ᵀ, and other type strains of the genus Lactiplantibacillus, constructed using (A) maximum-parsimony (MP) and (B) maximum-likelihood (ML) methods. Bootstrap values calculate from 1,000 replicates and are shown at the nodes; only values exceeding 70.0% are displayed. Oenococcus oeni JCM 6125T (GenBank accession no. AB022924) was used as the outgroup. Scale bars indicate 20 and 0.02 substitutions per nucleotide position for MP and ML, respectively.
jm-2507007-Supplementary-Fig-S1.pdf
Fig. S2.
Reconstructed metaobolic pathway from glucose to lactate fermentation in homofermentative and heterofermentation strains. Our strains (CBA3605T and CBA3606T; red), Lactiplantibacillus plantarum DSM 20174T (homofermentative reference; blue), and Leuconostoc mesenteroides ATCC 8293T (heterofermentative reference; green) were compared. Both strains CBA3605T and CBA3606T possessed the complete set of glycolytic genes, including phosphofructokinase-1 (EC 2.7.1.11) and fructose-bisphosphate aldolase (EC 4.1.2.13), similar to DSM 20174T, whereas Leu. mesenteroides ATCC 8293T lacked these key genes.
jm-2507007-Supplementary-Fig-S2.pdf
Fig. S3.
Scanning electron microscopy (SEM) images showing the cellular morphology of strains (A) CBA3605T and (B) CBA3606T grown on MRS agar at 30°C for 2 days. Scale bar 1 μm.
jm-2507007-Supplementary-Fig-S3.pdf
Fig. S4.
Thin-layer chromatograms (TLC) of amino acids from the peptidoglycan of CBA3605T, CBA3606T, and related type strains of the genus Lactiplantibacillus. Amino acids were extracted and hydrolyzed by 6 N HCl at 121°C for 15 min, and analyzed by thin-layer chromatography. Strains: 1, CBA3605T; 2, CBA3606T; 3, Lp. daoliensis NCIMB 15181T; 4, Lp. nangangensis NCIMB 15186T; 5, Lp. pingfangensis NCIMB 15187T; 6, Lp. brownii DSM 116485T.
jm-2507007-Supplementary-Fig-S4.pdf
Fig. S5.
Two-dimensional thin-layer chromatograms (TLC) of the polar lipids of CBA3605T and CBA3606T. Solvent systems: (I) chloroform-methanol-water (65:25:4, v/v/v); (II) chloroform-acetic acid-methanol-water (80:12:15:4, v/v/v/v). The TLC plates were sprayed with 10% ethanolic molybdatophosphoric acid, ninhydrin, and Dittmer-Lester reagents for the detection of total polar lipids. Abbreviations: DPG, diphosphatidylglycerol; PG, phosphatidylglycerol; PE, phosphatidylethanolamine; GL, unidentified glycolipid; L, unidentified lipid; PL, phospholipid; APL, aminophospholipid; APGL, aminophosphoglycolipid.
jm-2507007-Supplementary-Fig-S5.pdf
Table S1.
Distribution of Clusters of Orthologous Groups (COG) functional categories in strains CBA3605T, CBA3606T, their closely related type strains, and strain DSM 20174T of the genus Lactiplantibacillus (abbreviated as Lp.). The highest, second-highest, and third-highest gene counts for each strain are indicated in bold. Strains: 1, CBA3605T; 2, CBA3606T; 3, Lp. daoliensis 116-1AT; 4, Lp. nangangensis 381-7T; 5, Lp. pingfangensis 382-1T; 6, Lp. brownii WILCCON 0030T; 7, Lp. plantarum DMS 20174T (type species of the genus Lactiplantibacillus)
jm-2507007-Supplementary-Table-S1.pdf
Fig. 1.Phylogenetic tree constructed based on 16S rRNA gene sequences of strains CBA3605T, CBA3606T, and other type strains of the genus Lactiplantibacillus using the neighbor-joining method. Bootstrap values based on 1,000 replicates are shown at the nodes, but only those exceeding 70.0% are displayed for the neighbor-joining, maximum-parsimony, and maximum-likelihood methods. Filled circles indicate nodes supported by all three treeing methods. Levilactobacillus tangyuanensis 137-3T (MK110859) was used as the outgroup. Scale bar represents 0.01 substitutions per nucleotide position.
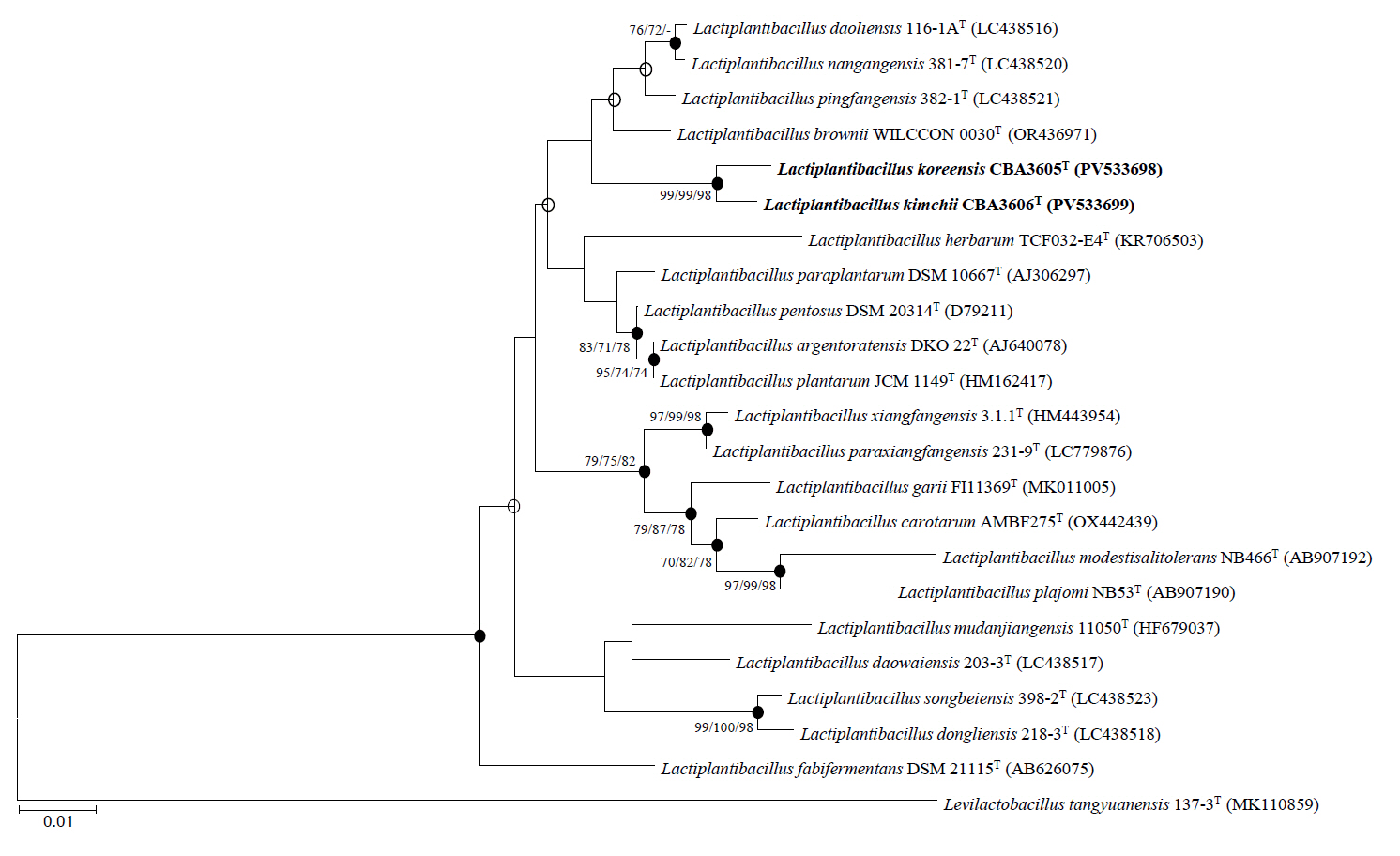
Fig. 2.Phylogenetic tree constructed based on whole genome sequences of strains CBA3605T, CBA3606T, and other type strains of the genus Lactiplantibacillus using the maximum-likelihood methods. Bootstrap values based on 1,000 replicates are shown at the nodes, but only those exceeding 70.0% are displayed. Levilactobacillus tangyuanensis 137-3T (RHOA00000000) was used as the outgroup. Scale bar represents 0.1 substitutions per sequence position.
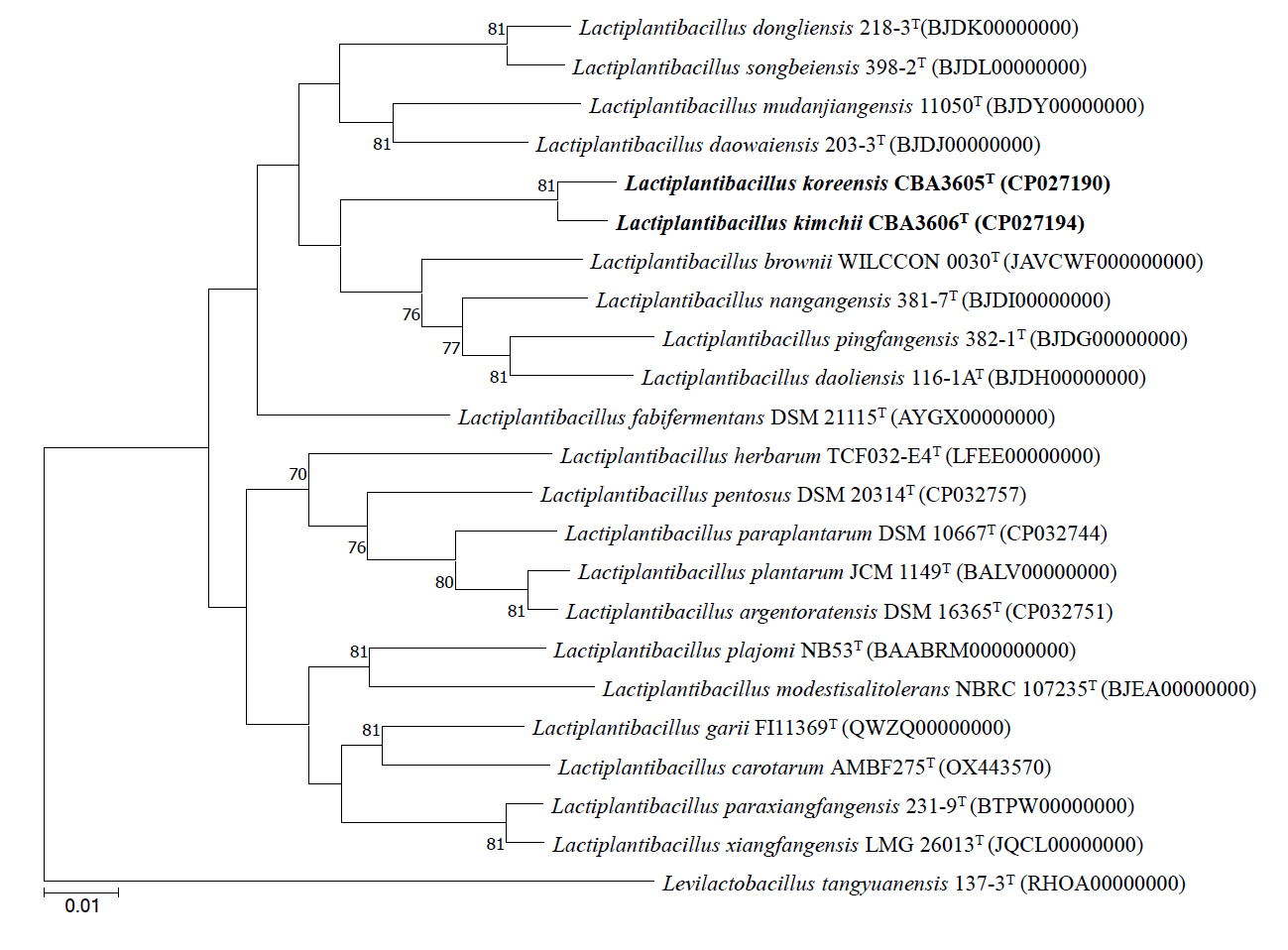
Fig. 3.Reconstructed pentose phosphate pathway of strains CBA3605T and CBA3606T. Pink and blue boxes indicate genes present in strain CBA3605T and CBA3606T, respectively, while white boxes denote the absence of the corresponding genes. Genes absent in both strains are not shown.
Table 1.General genomic features of strains CBA3605T, CBA3606T, their closely related type strains, and strain DSM 20174T of the genus Lactiplantibacillus (Lp.)
|
Characteristic |
1*
|
2*
|
3 |
4 |
5 |
6 |
7 |
|
Status† (Number of contigs) |
C (4) |
C (4) |
D (19) |
D (39) |
D (59) |
D (11) |
C (2) |
|
Genome size (bp) |
2,494,909 |
2,449,069 |
2,632,925 |
2,901,270 |
2,895,555 |
3,347,921 |
3,250,154 |
|
DNA G + C content (%) |
43.0 |
43.5 |
43.5 |
44.5 |
44.0 |
43.5 |
44.5 |
|
Number of genes |
2,497 |
2,493 |
2,466 |
2,779 |
2,733 |
3,187 |
3,086 |
|
Number of protein-coding genes |
2,362 |
2,330 |
2,385 |
2,711 |
2,656 |
3,030 |
2,962 |
|
Number of tRNA genes |
62 |
62 |
50 |
43 |
50 |
63 |
71 |
|
Number of pseudogenes |
54 |
82 |
25 |
18 |
19 |
74 |
33 |
|
Number of rRNA gene operons (5S, 16S, 23S) |
6, 5, 5 |
6, 5, 5 |
1, 1, 1 |
1, 1, 1 |
1, 2, 1 |
6, 5, 5 |
6,5,5 |
|
GenBank accession numbers |
CP027190–3 |
CP027194–7 |
BJDH |
BJDI |
BJDG |
JAVCWF00000000 |
NZ_CP039121–2 |
|
00000000 |
00000000 |
00000000 |
|
Completeness (%) |
98.5 |
99.0 |
99.4 |
99.0 |
99.4 |
99.0 |
99.0 |
|
Contamination (%) |
1.2 |
1.4 |
0.7 |
0.7 |
0.7 |
1.1 |
1.4 |
Table 2.Pair-wise average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) values of strains CBA3605T, CBA3606T, their closely related type strains, and strain DSM 20174T of the genus Lactiplantibacillus (Lp.); numbers below and to the left of the diagonal represent ANI values, while those above and to the right of the diagonal represent dDDH values.
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
1 |
- |
45.00 |
21.40 |
21.00 |
21.30 |
21.70 |
20.70 |
|
2 |
91.69 |
- |
21.00 |
20.90 |
21.30 |
21.50 |
21.00 |
|
3 |
76.92 |
76.52 |
- |
25.20 |
25.90 |
24.70 |
20.70 |
|
4 |
76.66 |
76.64 |
81.76 |
- |
24.40 |
24.60 |
21.10 |
|
5 |
76.62 |
76.65 |
82.38 |
80.81 |
- |
24.10 |
20.60 |
|
6 |
76.85 |
76.73 |
80.78 |
80.98 |
80.23 |
- |
21.70 |
|
7 |
74.86 |
74.91 |
75.03 |
74.97 |
74.71 |
75.12 |
- |
Table 3.Phenotypic characteristics of strains CBA3605T, CBA3606T, their closely related type strains, and strain DSM 20174T of the genus Lactiplantibacillus (Lp.)
|
Characteristic |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
Colony color |
Beige |
Beige |
Beige |
Beige |
Beige |
White |
N.D. |
|
Optimum growth at: |
|
|
|
|
|
|
|
|
pH (optimum) |
4.0–7.0 (5.0) |
4.0–7.0 (5.0) |
4.0–7.0 (6.0) |
4.0–7.0 (5.0) |
4.0–7.0 (5.0) |
4.0–7.0 (7.0) |
5.0–7.0 (N.D.) |
|
Temperature (optimum, °C) |
15–35 (30) |
15–35 (30) |
15–35 (30) |
15–35 (30) |
15–35 (30) |
15–35 (30) |
15–37 (N.D.) |
|
NaCl (optimum, %) |
0–6.0 (0) |
0–6.0 (0) |
0–4.0 (0) |
0–6.0 (0) |
0–4.0 (0) |
0–6.0 (0) |
0–8.0 (N.D.) |
|
API 50 CHL test: |
|
|
|
|
|
|
|
|
D-ribose |
+ |
+ |
- |
- |
+ |
+ |
+ |
|
L-rhamnose |
+ |
+ |
- |
- |
- |
- |
- |
|
D-sorbitol |
+ |
+ |
- |
- |
- |
- |
+ |
|
Gentiobiose |
+ |
+ |
+ |
- |
+ |
- |
+ |
|
D-raffinose |
- |
- |
- |
+ |
- |
- |
+ |
|
D-turanose |
- |
- |
- |
- |
+ |
- |
+ |
|
D-lactose |
+ |
- |
- |
- |
- |
- |
+ |
|
methyl-α-D-glucopyranoside |
- |
+ |
+ |
+ |
+ |
+ |
N.D. |
|
N-acetylglucosamine |
- |
+ |
+ |
+ |
+ |
+ |
+ |
Table 4.Cellular fatty acid composition (percentages) of strains CBA3605T, CBA3606T, and their closely related type strains of the genus Lactiplantibacillus (Lp.)
|
Fatty acid |
1 |
2 |
3 |
4 |
5 |
6 |
|
Saturated |
|
|
|
|
|
|
|
C10:0
|
- |
- |
- |
tr |
- |
tr |
|
C12:0
|
tr |
tr |
tr |
tr |
tr |
tr |
|
C14:0
|
tr |
tr |
tr |
tr |
tr |
tr |
|
C16:0
|
23.13 |
22.57 |
17.67 |
18.69 |
21.20 |
18.96 |
|
C17:0
|
tr |
tr |
tr |
tr |
tr |
tr |
|
C18:0
|
1.84 |
1.95 |
2.95 |
3.72 |
2.11 |
2.19 |
|
C19:0
|
- |
- |
tr |
- |
- |
- |
|
C20:0
|
tr |
tr |
20.86 |
5.72 |
tr |
tr |
|
Unsaturated |
|
|
|
|
|
|
|
C17:1 ω8c |
tr |
tr |
tr |
tr |
tr |
tr |
|
C18:1 ω9c |
32.40 |
30.29 |
21.97 |
29.87 |
39.43 |
40.20 |
|
C20:1 ω7c |
- |
- |
tr |
tr |
tr |
tr |
|
C20:1 ω9c |
- |
- |
tr |
tr |
- |
- |
|
Branched -chain fatty acid |
|
|
|
|
|
|
|
C10:0 iso |
tr |
tr |
- |
- |
- |
- |
|
C15:0 iso |
tr |
- |
tr |
- |
tr |
- |
|
C19:0 iso |
1.49 |
1.54 |
1.80 |
1.88 |
1.77 |
1.55 |
|
C19:1 iso I |
tr |
- |
tr |
tr |
tr |
tr |
|
Hydroxy fatty acids |
|
|
|
|
|
|
|
C17:0 2OH |
2.89 |
2.70 |
2.64 |
3.22 |
2.94 |
3.27 |
|
Other |
|
|
|
|
|
|
|
C16:0 N alcohol |
- |
- |
- |
0.11 |
- |
- |
|
Summed feature |
|
|
|
|
|
|
|
3 |
1.73 |
1.74 |
1.04 |
1.13 |
1.64 |
1.42 |
|
7 |
30.98 |
33.79 |
25.60 |
29.46 |
24.09 |
26.56 |
|
8 |
3.70 |
3.71 |
3.95 |
4.39 |
4.16 |
3.93 |
References
- Agarwal S, Sharma K, Swanson B, Yüksel GÜ, Clark S. 2006. Nonstarter lactic acid bacteria biofilms and calcium lactate crystals in cheddar cheese. J Dairy Sci. 89: 1452–1466. ArticlePubMed
- Burland TG. 1999. DNASTAR’s lasergene sequence analysis software. In Misener S, Krawetz SA. (eds.), Bioinformatics Methods and Protocols, pp. 71-91. Humana Totowa
- Cantalapiedra CP, Hernández-Plaza A, Letunic I, Bork P, Huerta-Cepas J. 2021. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol Biol Evol. 38: 5825–5829. ArticlePubMedPMCPDF
- Cappuccino JG, Sherman N. 2008. Microbiology: a laboratory manual, 5th edn. San Francisco: Pearson/Benjamin Cummings
- Chun BH, Kim KH, Jeon HH, Lee SH, Jeon CO. 2017. Pan-genomic and transcriptomic analyses of Leuconostoc mesenteroides provide insights into its genomic and metabolic features and roles in kimchi fermentation. Sci Rep. 7: 11504.ArticlePubMedPMCPDF
- Curk MC, Hubert JC, Bringel F. 1996. Lactobacillus paraplantarum sp. nov., a new species related to Lactobacillus plantarum. Int J Syst Evol Microbiol. 46: 595–598. Article
- De Bruyne K, Camu N, De Vuyst L, Vandamme P. 2009. Lactobacillus fabifermentans sp. nov. and Lactobacillus cacaonum sp. nov., isolated from Ghanaian cocoa fermentations. Int J Syst Evol Microbiol. 59: 7–12. ArticlePubMed
- Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 39: 783–791. ArticlePubMedLink
- Gomori G. 2010. Preparation of buffers for use in enzyme studies. In Lundblad R, Macdonald F. (eds.), Handbook of biochemistry and molecular biology, 4th edn, pp. 721–724. CRC Press
- Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, et al. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 57: 81–91. ArticlePubMed
- Heng YC, Silvaraju S, Lee JKY, Kittelmann S. 2023. Lactiplantibacillus brownii sp. nov., a novel psychrotolerant species isolated from sauerkraut. Int J Syst Evol Microbiol. 73: 006194.Article
- Kanehisa M, Sato Y, Morishima K. 2016. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol. 428: 726–731. ArticlePubMed
- Kent DJ, Chauhan K, Boor KJ, Wiedmann M, Martin NH. 2016. Spore test parameters matter: mesophilic and thermophilic spore counts detected in raw milk and dairy powders differ significantly by test method. J Dairy Sci. 99: 5180–5191. ArticlePubMed
- Kim J, Na SI, Kim D, Chun J. 2021. UBCG2: up-to-date bacterial core genes and pipeline for phylogenomic analysis. J Microbiol. 59: 609–618. ArticlePubMedPDF
- Kim M, Oh HS, Park SC, Chun J. 2014. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 64: 346–351. ArticlePubMed
- Lane DJ. 1991. 16S/23S rRNA sequencing. In Stackebrandt E, Goodfellow M. (eds.), Nucleic acid techniques in bacterial systematics, pp. 115–175. John Wiley and Sons
- Lee KW, Kim GS, Baek AH, Hwang HS, Kwon DY, et al. 2020. Isolation and characterization of kimchi starters Leuconostoc mesenteroides PBio03 and Leuconostoc mesenteroides PBio104 for manufacture of commercial kimchi. J Microbiol Biotechnol. 30: 1060–1066. ArticlePubMedPMC
- Lee I, Kim YO, Park SC, Chun J. 2016. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 66: 1100–1103. ArticlePubMed
- Liu DD, Gu CT. 2019. Lactobacillus pingfangensis sp. nov., Lactobacillus daoliensis sp. nov., Lactobacillus nangangensis sp. nov., Lactobacillus daowaiensis sp. nov., Lactobacillus dongliensis sp. nov., Lactobacillus songbeiensis sp. nov. and Lactobacillus kaifaensis sp. nov., isolated from traditional Chinese pickle. Int J Syst Evol Microbiol. 69: 3237–3247. ArticlePubMed
- Mao Y, Chen M, Horvath P. 2015. Lactobacillus herbarum sp. nov., a species related to Lactobacillus plantarum. Int J Syst Evol Microbiol. 65: 4682–4688. ArticlePubMed
- Miller L, Berger T. 1985. Bacteria identification by gas chromatography of whole cell fatty acids. Hewlett-Packard application note 228-41, pp. 1–8. Hewlett-Packard
- Minnikin DE, Patel PV, Alshamaony L, Goodfellow M. 1977. Polar lipid composition in the classification of Nocardia and related bacteria. Int J Syst Evol Microbiol. 27: 104–117. Article
- Orla-Jensen S. 1919. The lactic acid bacteria. Andr Fred Høst
- Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25: 1043–1055. ArticlePubMedPMC
- Price MN, Dehal PS, Arkin AP. 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 26: 1641–1650. ArticlePubMedPMC
- Sasser M. 1990. Identification of bacteria by gas chromatography of cellular fatty acids. MIDI technical note 101. MIDI Inc
- Schleifer KH, Kandler O. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 36: 407–477. ArticlePubMedPMCLink
- Tamura K, Stecher G, Kumar S. 2021. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 38: 3022–3027. ArticlePubMedPMCPDF
- Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, et al. 2016. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 44: 6614–6624. ArticlePubMedPMC
- Wang Y, Wu J, Lv M, Shao Z, Hungwe M, et al. 2021. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front Bioeng Biotechnol. 9: 612285.ArticlePubMedPMC
- Whittenbury R. 1964. Hydrogen peroxide formation and catalase activity in the lactic acid bacteria. J Gen Microbiol. 35: 13–26. ArticlePubMed
- Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, et al. 2017. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 67: 1613–1617. ArticlePubMedPMC
- Zheng J, Wittouck S, Salvetti E, Franz CMAP, Harris HMB, et al. 2020. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 70: 2782–2858. ArticlePubMed
Citations
Citations to this article as recorded by





 ePub Link
ePub Link Cite this Article
Cite this Article
 MSK
MSK