Articles
- Page Path
- HOME > J. Microbiol > Volume 63(11); 2025 > Article
-
Full article
Inhibition of cardiolipin biosynthesis partially suppresses the sensitivity of an Escherichia coli mutant lacking OmpC to envelope stress - Dae-Beom Ryu†, Umji Choi†, Gyubin Han, Chang-Ro Lee*
-
Journal of Microbiology 2025;63(11):e2507004.
DOI: https://doi.org/10.71150/jm.2507004
Published online: November 30, 2025
Department of Biological Sciences, Myongji University, Yongin 17058, Republic of Korea
- *Correspondence Chang-Ro Lee crlee@mju.ac.kr
- †These authors contributed equally to this work.
• Received: July 9, 2025 • Revised: September 2, 2025 • Accepted: September 2, 2025
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,420 Views
- 29 Download
ABSTRACT
- Porins in the outer membrane (OM) of Gram-negative bacteria play two main functions: passage of various extracellular molecules and maintenance of membrane integrity. OmpC, a non-specific porin, is involved in both functions; however, the exact mechanism of maintenance of membrane integrity remains unknown. In this study, we found that inhibiting cardiolipin biosynthesis partially restored the growth defect of the ompC mutant under envelope stress. Among the three enzymes involved in cardiolipin biosynthesis, ClsABC, this effect is primarily associated with ClsA. Notably, the deletion of ClsA also suppressed the similar phenotypes of an Escherichia coli mutant lacking YhdP, a transmembrane protein involved in phospholipid transport from the inner membrane to the OM. Collectively, these results imply that OmpC may contribute to membrane integrity, partially through mechanisms linked to transport or biosynthesis of phospholipids such as cardiolipin.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
This work was supported by a research grant from Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-RS-2023-00246684).
Supplementary Information
Fig. S1.
Table S1.
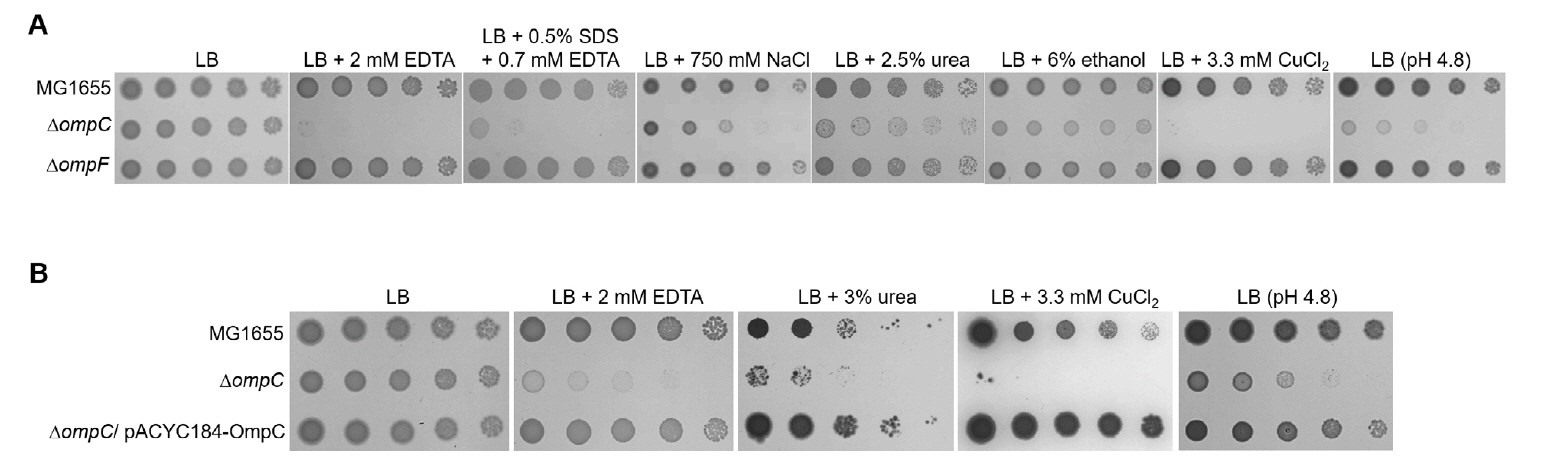
Fig. 1.The sensitivity of the ompC mutant to various envelope stresses. (A) The effect of depletion of OmpC on overcoming envelope stresses. (B) Complementation of the sensitivity of the ompC mutant to envelope stresses. (A and B) The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto an LB plate, LB plates containing indicated materials, or an LB plate adjusted to pH 4.8. All experiments were performed in triplicate, and a representative image is presented.
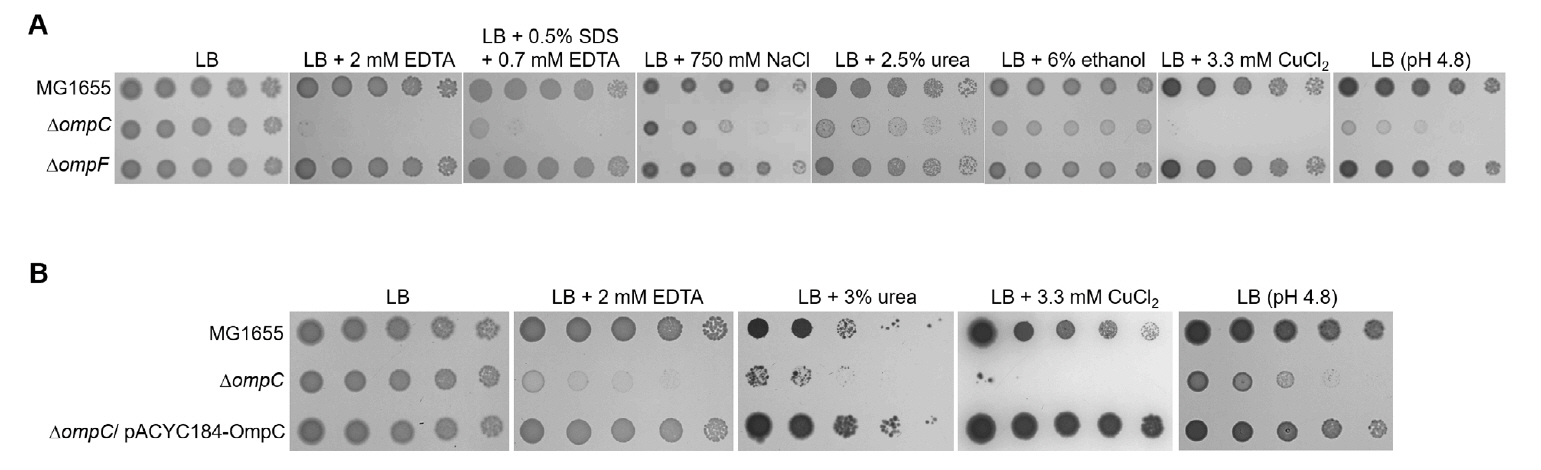

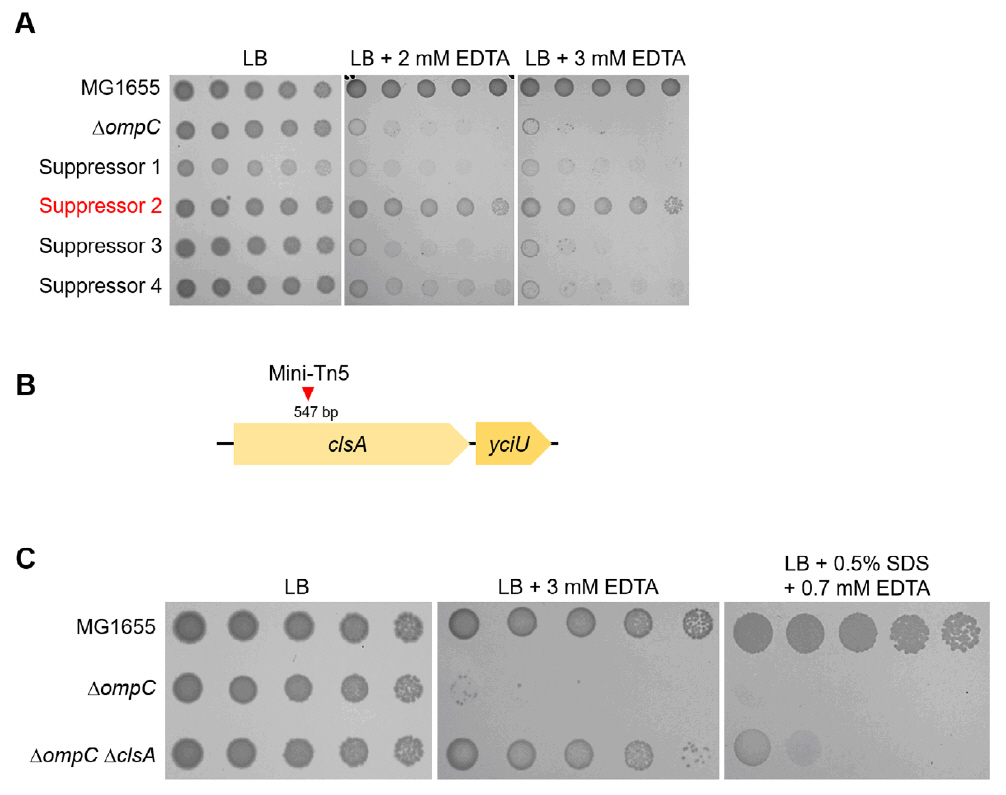
Fig. 2.The effect of the cardiolipin synthase ClsA on the sensitivity of the ompC mutant to envelope stresses. (A) Isolation of the suppressor mutant of the ompC mutant. The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto LB plates with or without the indicated concentrations of EDTA. (B) Schematic representation of a mini-Tn5 insertion site. The mini-Tn5 insertion site of a suppressor mutant (suppressor 2) has been indicated using a red arrow. (C) The depletion of ClsA partially suppresses the sensitivity of the ompC mutant to envelope stresses. The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto LB plates with or without the indicated materials. The experiments were performed in triplicate, and a representative image is presented.
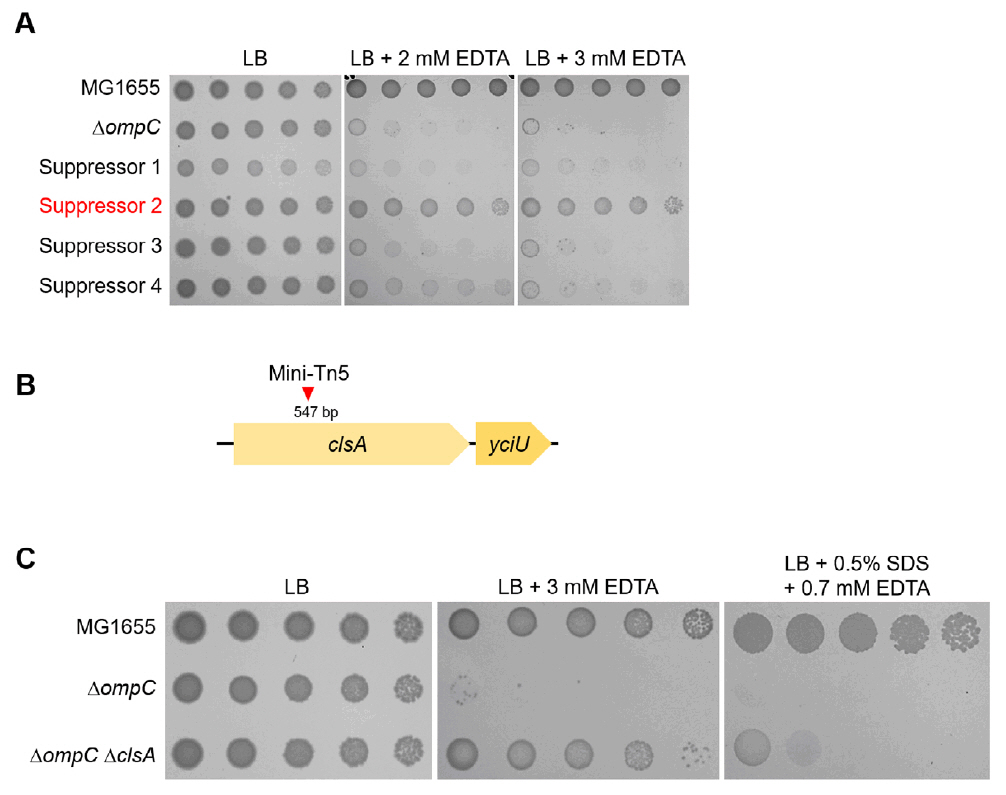

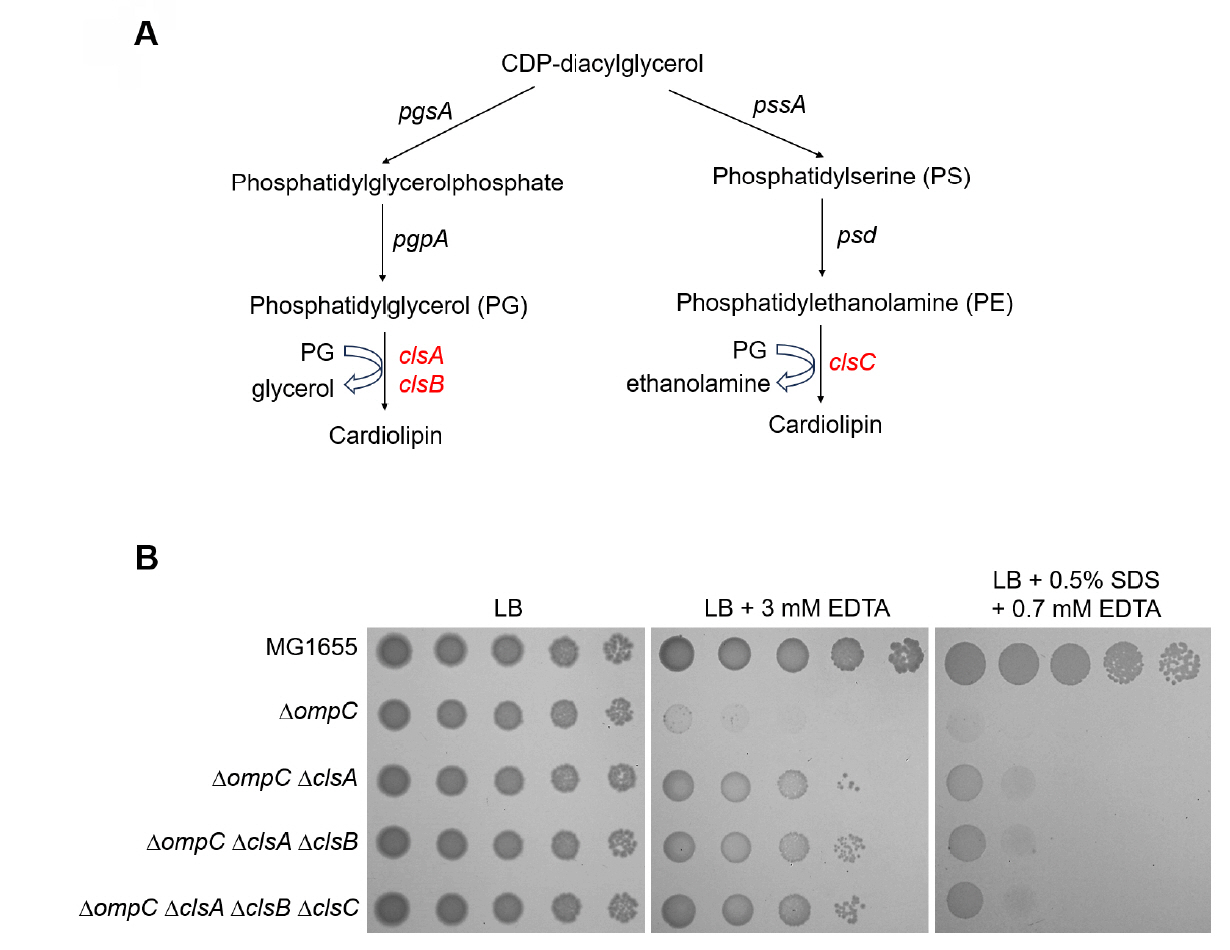
Fig. 3.The effect of other cardiolipin synthases, ClsB and ClsC, on the sensitivity of the ompC mutant to envelope stresses. (A) Schematic representation depicting two distinct pathways of cardiolipin biosynthesis. ClsA and its isozyme ClsB synthesize cardiolipin through catalyzing the condensation of two PGs with a concomitant release of glycerol. ClsC synthesizes cardiolipin through catalyzing the condensation of PG and PE with a concomitant release of ethanolamine. (B) Additional depletions of ClsB and ClsC hardly affect suppression of the sensitivity of the ompC mutant to envelope stresses. The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto LB plates with or without the indicated materials. The experiments were performed in triplicate, and a representative image is presented.
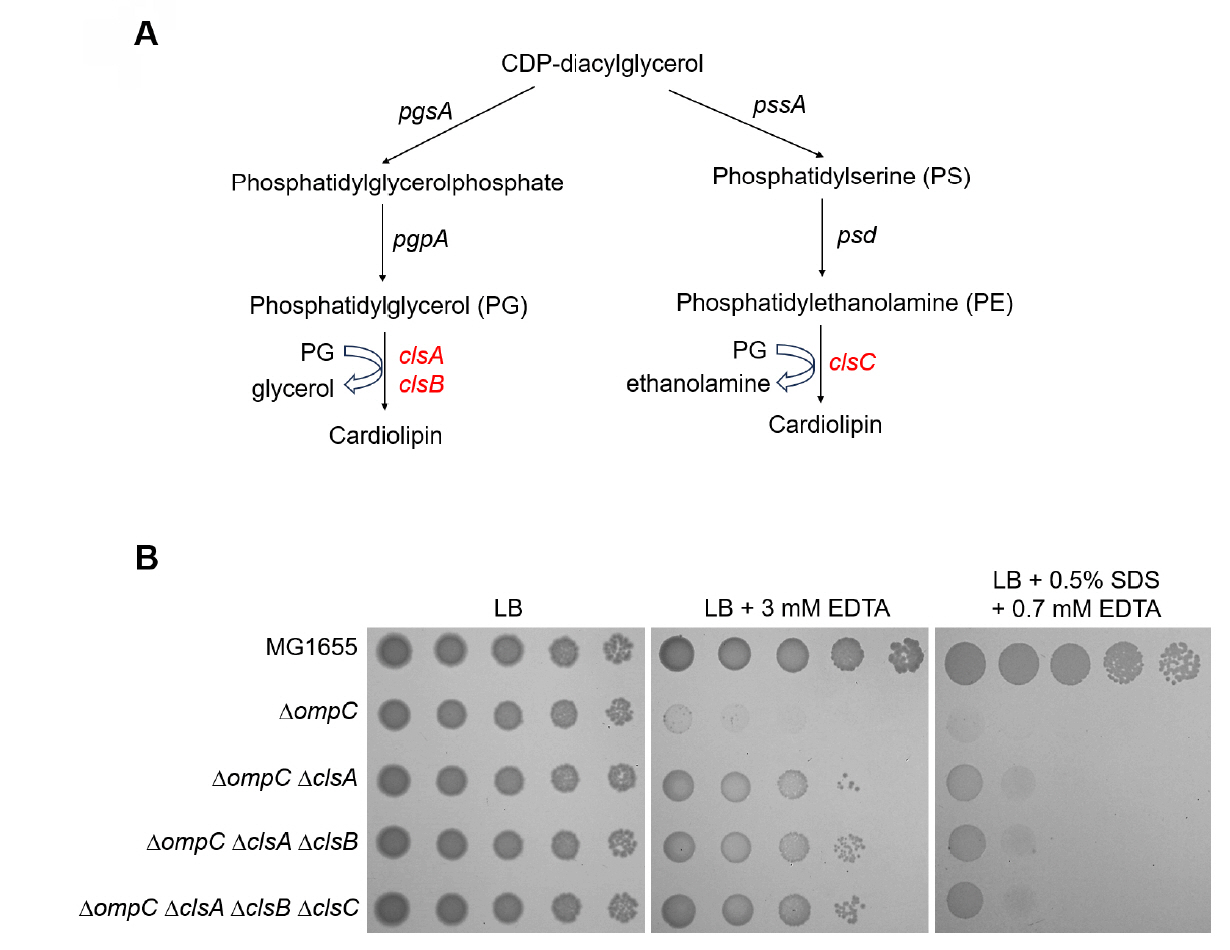

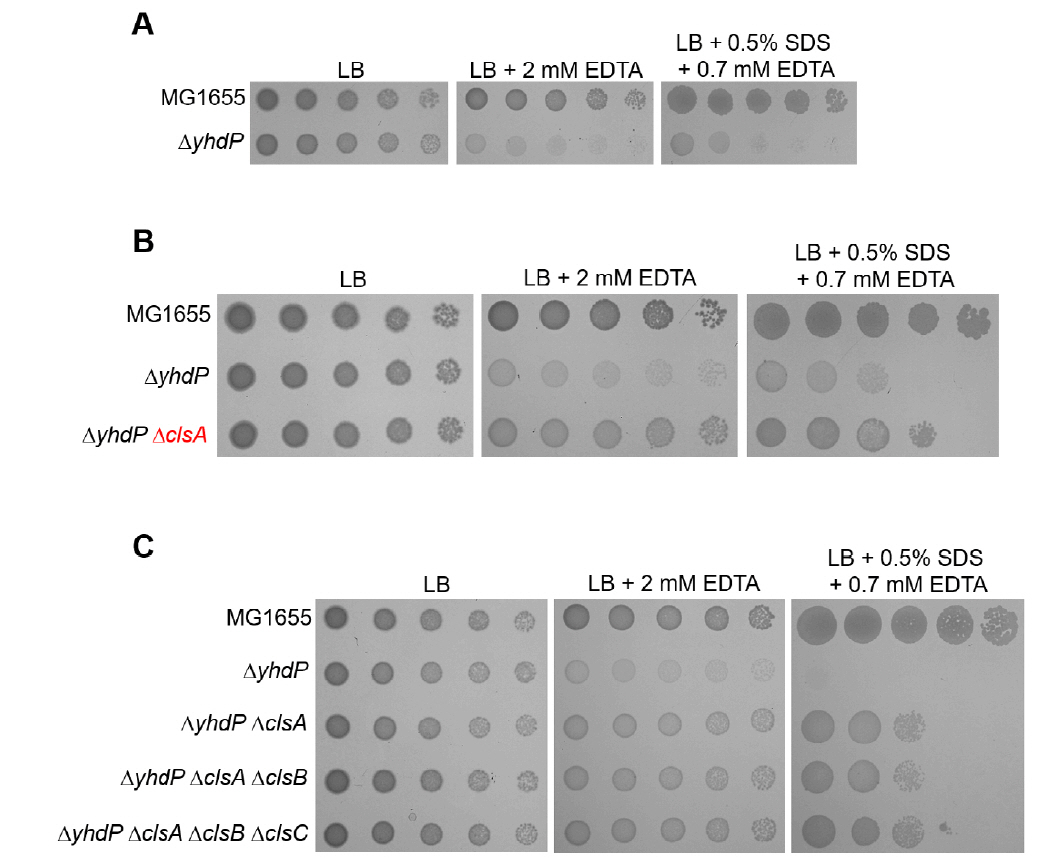
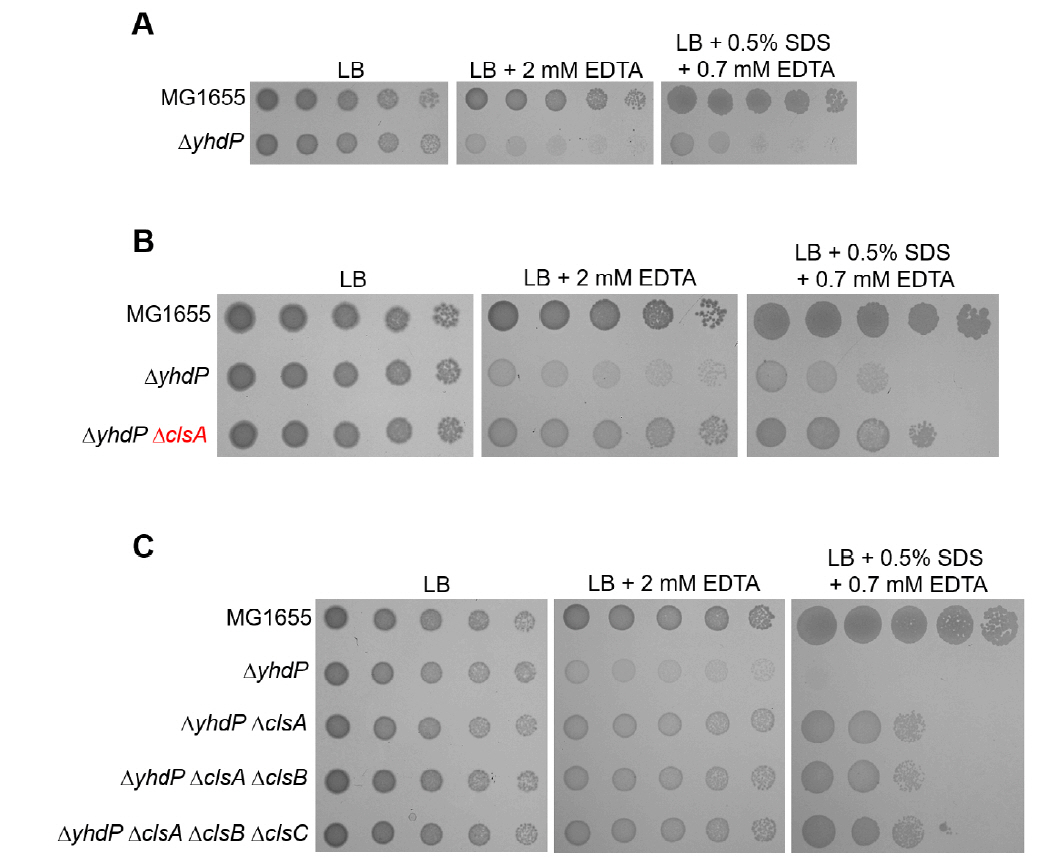
Fig. 4.The effect of the cardiolipin synthase ClsA on the sensitivity of the yhdP mutant to envelope stresses. (A) The effect of depletion of YhdP on overcoming envelope stresses. (B) The depletion of ClsA partially suppresses the sensitivity of the yhdP mutant to envelope stresses. (C) Additional depletions of ClsB and ClsC hardly affect suppression of the sensitivity of the yhdP mutant to envelope stresses. (A–C) The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto LB plates with or without the indicated materials. The experiments were performed in triplicate, and a representative image is presented.

- Baslé A, Rummel G, Storici P, Rosenbusch JP, Schirmer T. 2006. Crystal structure of osmoporin OmpC from E. coli at 2.0 Å. J Mol Biol. 362: 933–942. ArticlePubMed
- Boags AT, Samsudin F, Khalid S. 2019. Binding from both sides: TolR and full-length OmpA bind and maintain the local structure of the E. coli cell wall. Structure. 27: 713–724.e2. ArticlePubMed
- Choi BJ, Choi U, Ryu DB, Lee CR. 2024. PhoPQ-mediated lipopolysaccharide modification governs intrinsic resistance to tetracycline and glycylcycline antibiotics in Escherichia coli. mSystems. 9: e00964-24. ArticlePubMedPMCLink
- Choi U, Lee CR. 2019. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front Microbiol. 10: 953.ArticlePubMedPMC
- Choi U, Park SH, Lee HB, Son JE, Lee CR. 2023. Coordinated and distinct roles of peptidoglycan carboxypeptidases DacC and DacA in cell growth and shape maintenance under stress conditions. Microbiol Spectr. 11: e00014–23. ArticlePubMedPMCLink
- Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 97: 6640–6645. ArticlePubMedPMC
- DeChavigny A, Heacock PN, Dowhan W. 1991. Sequence and inactivation of the pss gene of Escherichia coli. Phosphatidylethanolamine may not be essential for cell viability. J Biol Chem. 266: 5323–5332. ArticlePubMed
- Douglass MV, Cléon F, Trent MS. 2021. Cardiolipin aids in lipopolysaccharide transport to the Gram-negative outer membrane. Proc Natl Acad Sci USA. 118: e2018329118. ArticlePubMedPMC
- Douglass MV, McLean AB, Trent MS. 2022. Absence of YhdP, TamB, and YdbH leads to defects in glycerophospholipid transport and cell morphology in Gram-negative bacteria. PLoS Genet. 18: e1010096. ArticlePubMedPMC
- Goodall ECA, Robinson A, Johnston IG, Jabbari S, Turner KA, et al. 2018. The essential genome of Escherichia coli K-12. mBio. 9: e02096–17. ArticlePubMedPMCLink
- Gorzelak P, Klein G, Raina S. 2021. Molecular basis of essentiality of early critical steps in the lipopolysaccharide biogenesis in Escherichia coli K-12: requirement of MsbA, cardiolipin, LpxL, LpxM and GcvB. Int J Mol Sci. 22: 5099.ArticlePubMedPMC
- Killian JA, Koorengevel MC, Bouwstra JA, Gooris G, Dowhan W, et al. 1994. Effect of divalent cations on lipid organization of cardiolipin isolated from Escherichia coli strain AH930. Biochim Biophys Acta. 1189: 225–232. ArticlePubMed
- Larsen RA, Wilson MM, Guss AM, Metcalf WW. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch Microbiol. 178: 193–201. ArticlePubMedPDF
- Lee HB, Park SH, Lee CR. 2021. The inner membrane protein LapB is required for adaptation to cold stress in an LpxC-independent manner. J Microbiol. 59: 666–674. ArticlePubMedPDF
- Mandela E, Stubenrauch CJ, Ryoo D, Hwang H, Cohen EJ, et al. 2022. Adaptation of the periplasm to maintain spatial constraints essential for cell envelope processes and cell viability. eLife. 11: e73516. ArticlePubMedPMCPDF
- Mitchell AM, Silhavy TJ. 2019. Envelope stress responses: balancing damage repair and toxicity. Nat Rev Microbiol. 17: 417–428. ArticlePubMedPMCPDF
- Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 67: 593–656. ArticlePubMedPMCLink
- Nikaido H, Rosenberg EY, Foulds J. 1983. Porin channels in Escherichia coli: studies with b-lactams in intact cells. J Bacteriol. 153: 232–240. ArticlePubMedPMCLink
- Nishijima S, Asami Y, Uetake N, Yamagoe S, Ohta A, et al. 1988. Disruption of the Escherichia coli cls gene responsible for cardiolipin synthesis. J Bacteriol. 170: 775–780. ArticlePubMedPMCLink
- Pagès JM, James CE, Winterhalter M. 2008. The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol. 6: 893–903. ArticlePubMedPDF
- Pauptit RA, Zhang H, Rummel G, Schirmer T, Jansonius JN, et al. 1991. Trigonal crystals of porin from Escherichia coli. J Mol Biol. 218: 505–507. ArticlePubMed
- Pluschke G, Hirota Y, Overath P. 1978. Function of phospholipids in Escherichia coli: characterization of a mutant deficient in cardiolipin synthesis. J Biol Chem. 253: 5048–5055. ArticlePubMed
- Rai AK, Sawasato K, Bennett HC, Kozlova A, Sparagna GC, et al. 2024. Genetic evidence for functional diversification of Gram-negative intermembrane phospholipid transporters. PLoS Genet. 20: e1011335. ArticlePubMedPMC
- Ruiz N, Davis RM, Kumar S. 2021. YhdP, TamB, and YdbH are redundant but essential for growth and lipid homeostasis of the Gram-negative outer membrane. mBio. 12: e02714–21. ArticlePubMedPMCLink
- Shibuya I, Miyazaki C, Ohta A. 1985. Alteration of phospholipid composition by combined defects in phosphatidylserine and cardiolipin synthases and physiological consequences in Escherichia coli. J Bacteriol. 161: 1086–1092. ArticlePubMedPMCLink
- Son JE, Park SH, Choi U, Lee CR. 2024. Lytic transglycosylase repertoire diversity enables intrinsic antibiotic resistance and daughter cell separation in Escherichia coli under acidic stress. Antimicrob Agents Chemother. 68: e0037224. ArticlePubMedPMCLink
- Sung CG, Choi U, Lee CR. 2020. Phenotypic characterization of a conserved inner membrane protein YhcB in Escherichia coli. J Microbiol. 58: 598–605. ArticlePubMedPDF
- Tan BK, Bogdanov M, Zhao J, Dowhan W, Raetz CRH, et al. 2012. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc Natl Acad Sci USA. 109: 16504–16509. ArticlePubMedPMC
- Tan WB, Chng SS. 2024. How bacteria establish and maintain outer membrane lipid asymmetry. Annu Rev Microbiol. 78: 553–573. ArticlePubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

Inhibition of cardiolipin biosynthesis partially suppresses the sensitivity of an Escherichia coli mutant lacking OmpC to envelope stress
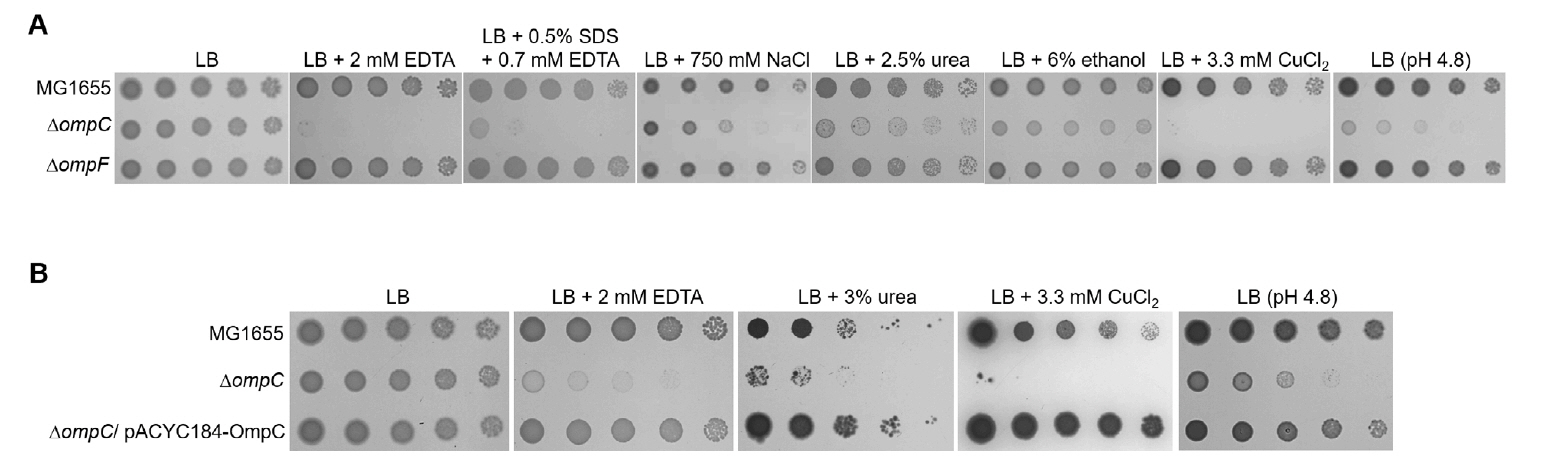
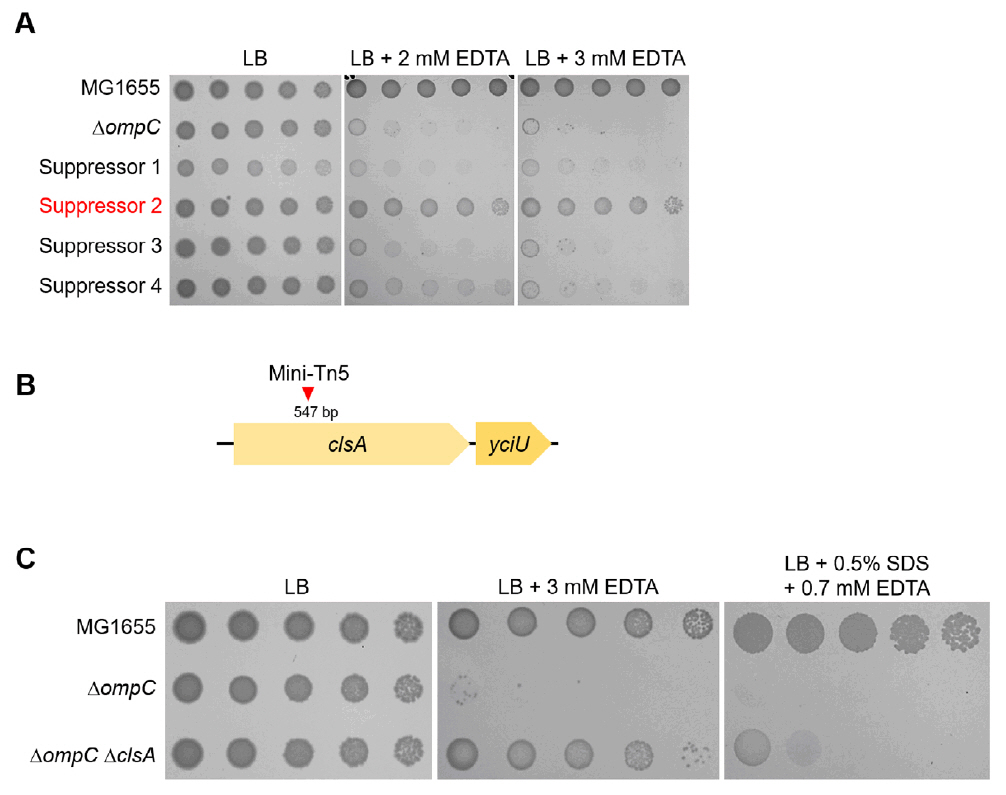
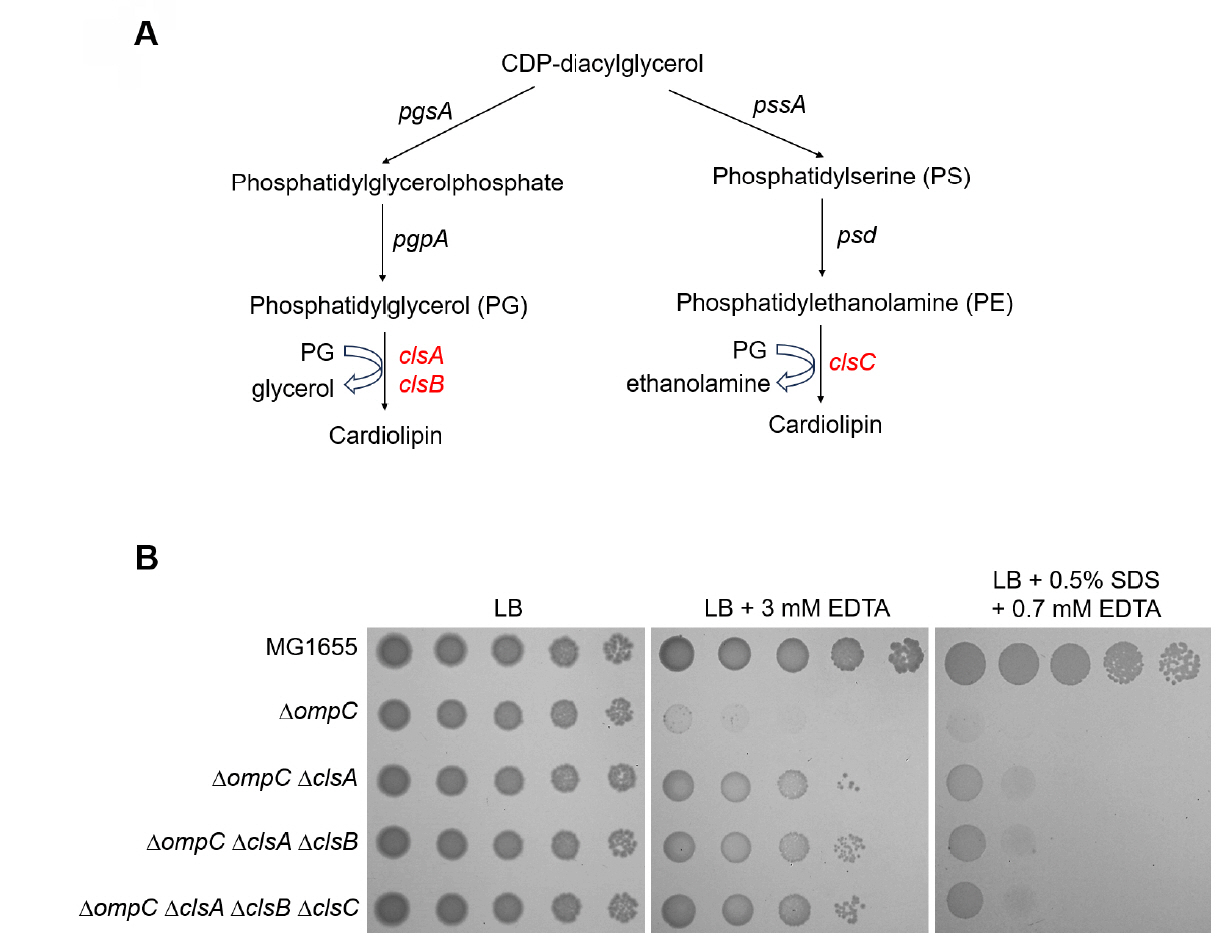
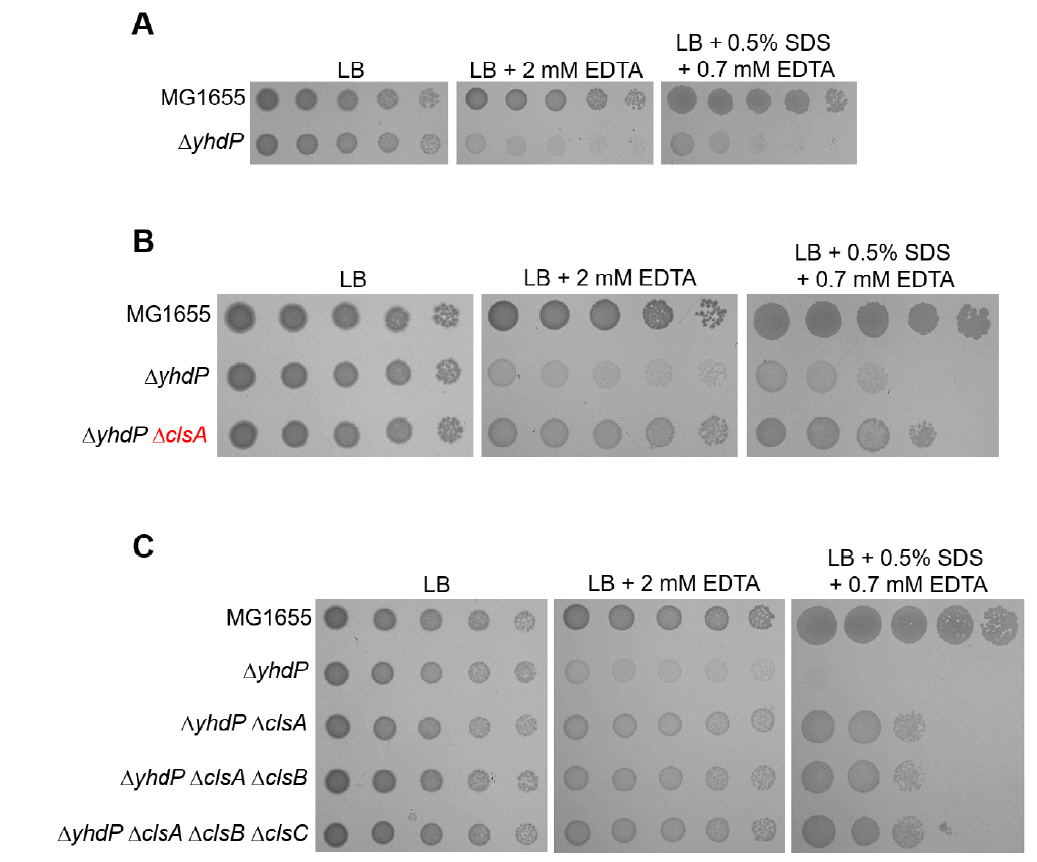
Fig. 1. The sensitivity of the ompC mutant to various envelope stresses. (A) The effect of depletion of OmpC on overcoming envelope stresses. (B) Complementation of the sensitivity of the ompC mutant to envelope stresses. (A and B) The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto an LB plate, LB plates containing indicated materials, or an LB plate adjusted to pH 4.8. All experiments were performed in triplicate, and a representative image is presented.
Fig. 2. The effect of the cardiolipin synthase ClsA on the sensitivity of the ompC mutant to envelope stresses. (A) Isolation of the suppressor mutant of the ompC mutant. The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto LB plates with or without the indicated concentrations of EDTA. (B) Schematic representation of a mini-Tn5 insertion site. The mini-Tn5 insertion site of a suppressor mutant (suppressor 2) has been indicated using a red arrow. (C) The depletion of ClsA partially suppresses the sensitivity of the ompC mutant to envelope stresses. The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto LB plates with or without the indicated materials. The experiments were performed in triplicate, and a representative image is presented.
Fig. 3. The effect of other cardiolipin synthases, ClsB and ClsC, on the sensitivity of the ompC mutant to envelope stresses. (A) Schematic representation depicting two distinct pathways of cardiolipin biosynthesis. ClsA and its isozyme ClsB synthesize cardiolipin through catalyzing the condensation of two PGs with a concomitant release of glycerol. ClsC synthesizes cardiolipin through catalyzing the condensation of PG and PE with a concomitant release of ethanolamine. (B) Additional depletions of ClsB and ClsC hardly affect suppression of the sensitivity of the ompC mutant to envelope stresses. The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto LB plates with or without the indicated materials. The experiments were performed in triplicate, and a representative image is presented.
Fig. 4. The effect of the cardiolipin synthase ClsA on the sensitivity of the yhdP mutant to envelope stresses. (A) The effect of depletion of YhdP on overcoming envelope stresses. (B) The depletion of ClsA partially suppresses the sensitivity of the yhdP mutant to envelope stresses. (C) Additional depletions of ClsB and ClsC hardly affect suppression of the sensitivity of the yhdP mutant to envelope stresses. (A–C) The cells of the indicated strains were serially diluted from 108 to 104 cells/ml in 10-fold steps and spotted onto LB plates with or without the indicated materials. The experiments were performed in triplicate, and a representative image is presented.
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Inhibition of cardiolipin biosynthesis partially suppresses the sensitivity of an Escherichia coli mutant lacking OmpC to envelope stress
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article