Articles
- Page Path
- HOME > J. Microbiol > Volume 63(11); 2025 > Article
-
Full article
Encapsulin protein MAV2054 enhances Mycobacterium avium virulence by promoting Cdc42-dependent epithelial cell invasion - Dong Ho Kim1,2,3, I Jeong Jo1,3,4, Min Ju Kang1,3,4, Yi Seol Kim1,3,4, Duyen Do Tran Huong1,3, Kyungho Woo1,2,3, Ho-Sung Park1,3,4, Hwa-Jung Kim1,2,3, Chul Hee Choi1,2,3,4,*
-
Journal of Microbiology 2025;63(11):e2506008.
DOI: https://doi.org/10.71150/jm.2506008
Published online: November 30, 2025
1Department of Microbiology, School of Medicine, Chungnam National University, Daejeon 35015, Republic of Korea
2Translational Immunology Institute, School of Medicine, Chungnam National University, Daejeon 35015, Republic of Korea
3Department of Medical Science, School of Medicine, Chungnam National University, Daejeon 35015, Republic of Korea
4System Network Inflammation Control Research Center, School of Medicine, Chungnam National University, Daejeon 35015, Republic of Korea
- *Correspondence Chul Hee Choi choich@cnu.ac.kr
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,149 Views
- 35 Download
ABSTRACT
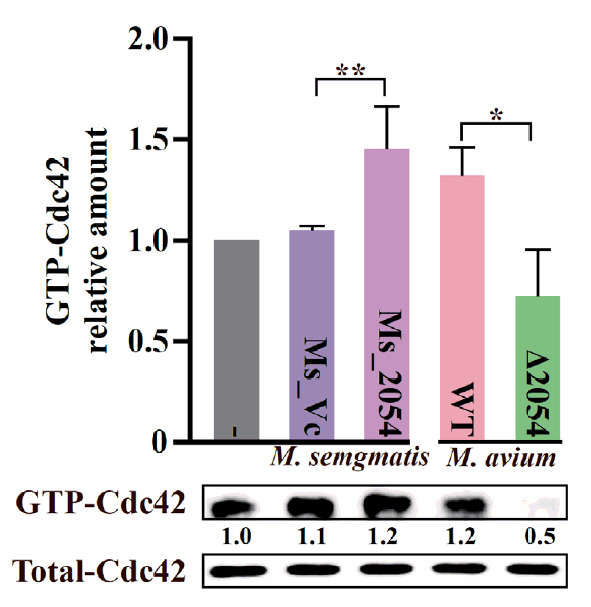
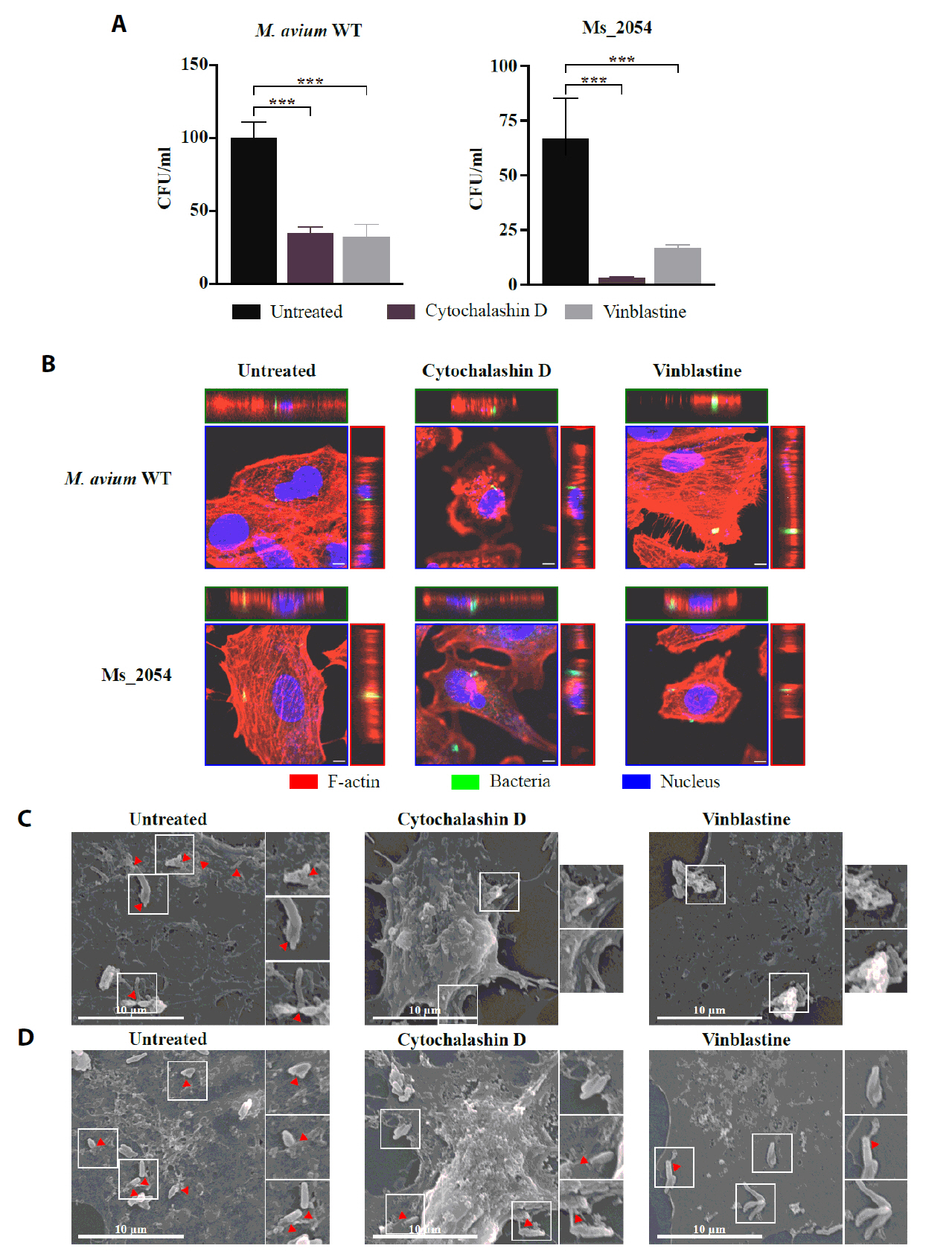
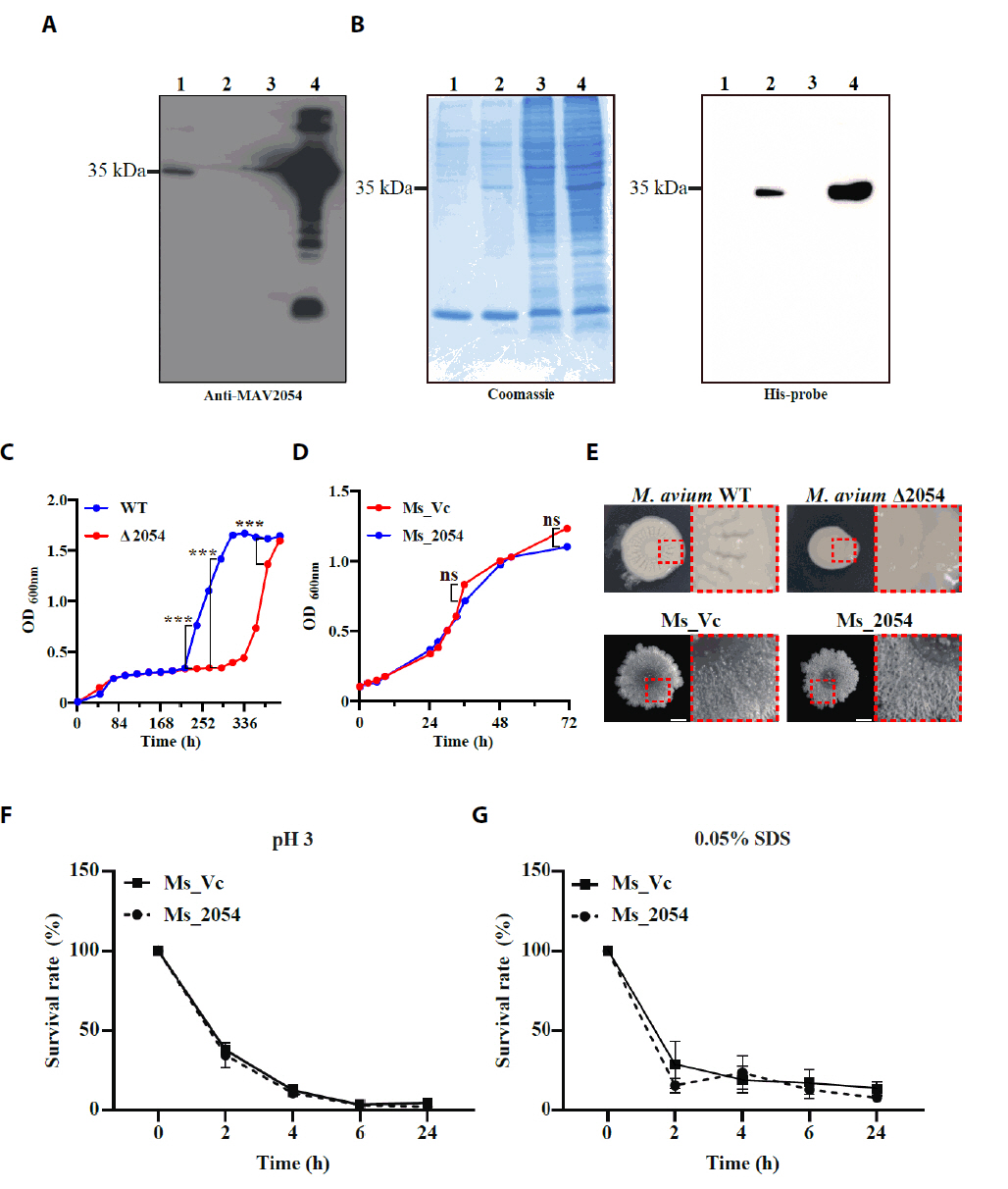
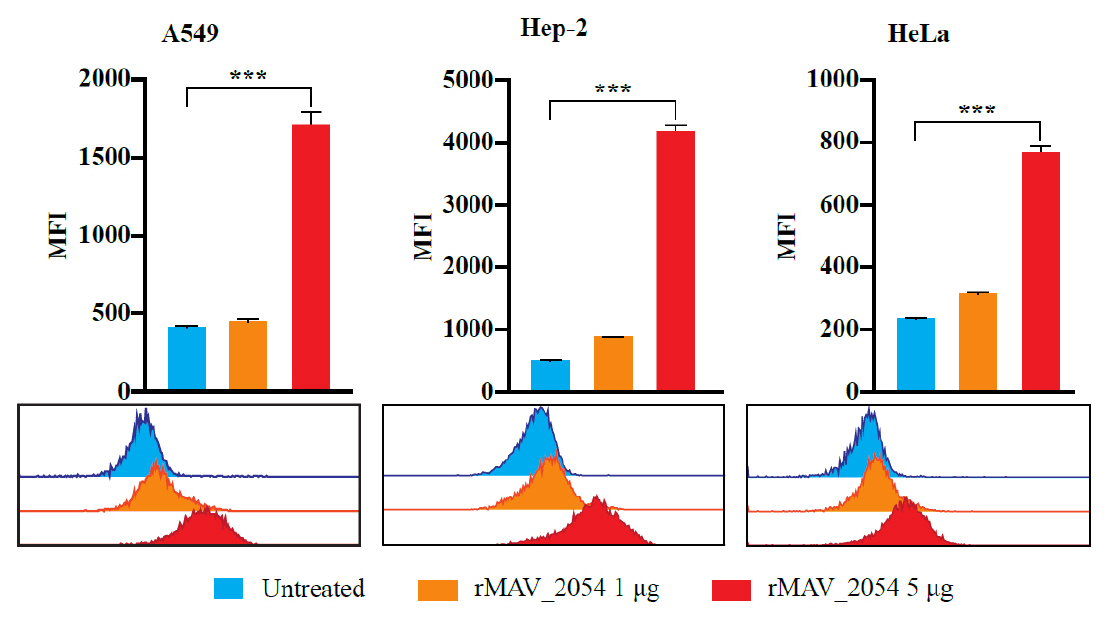
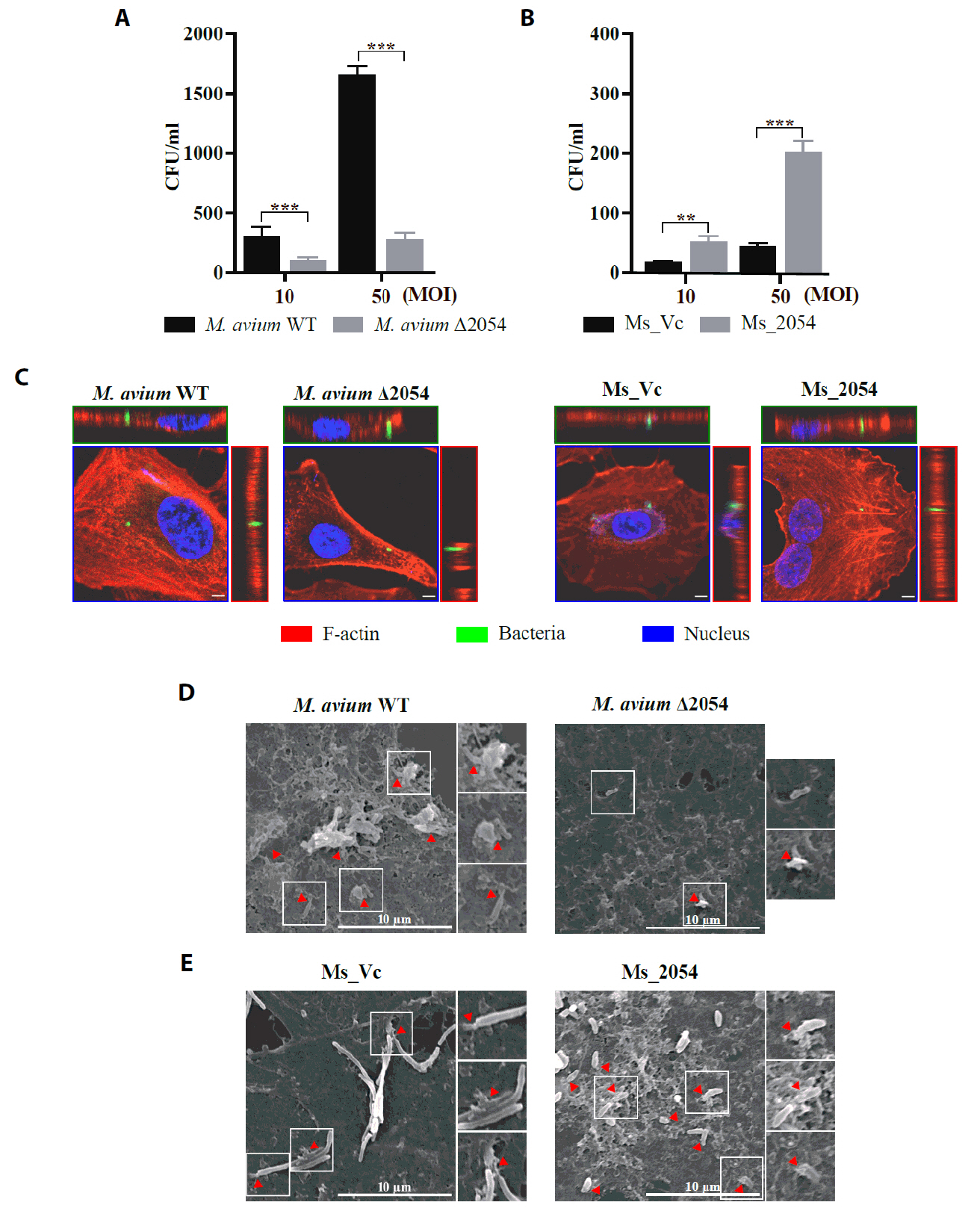
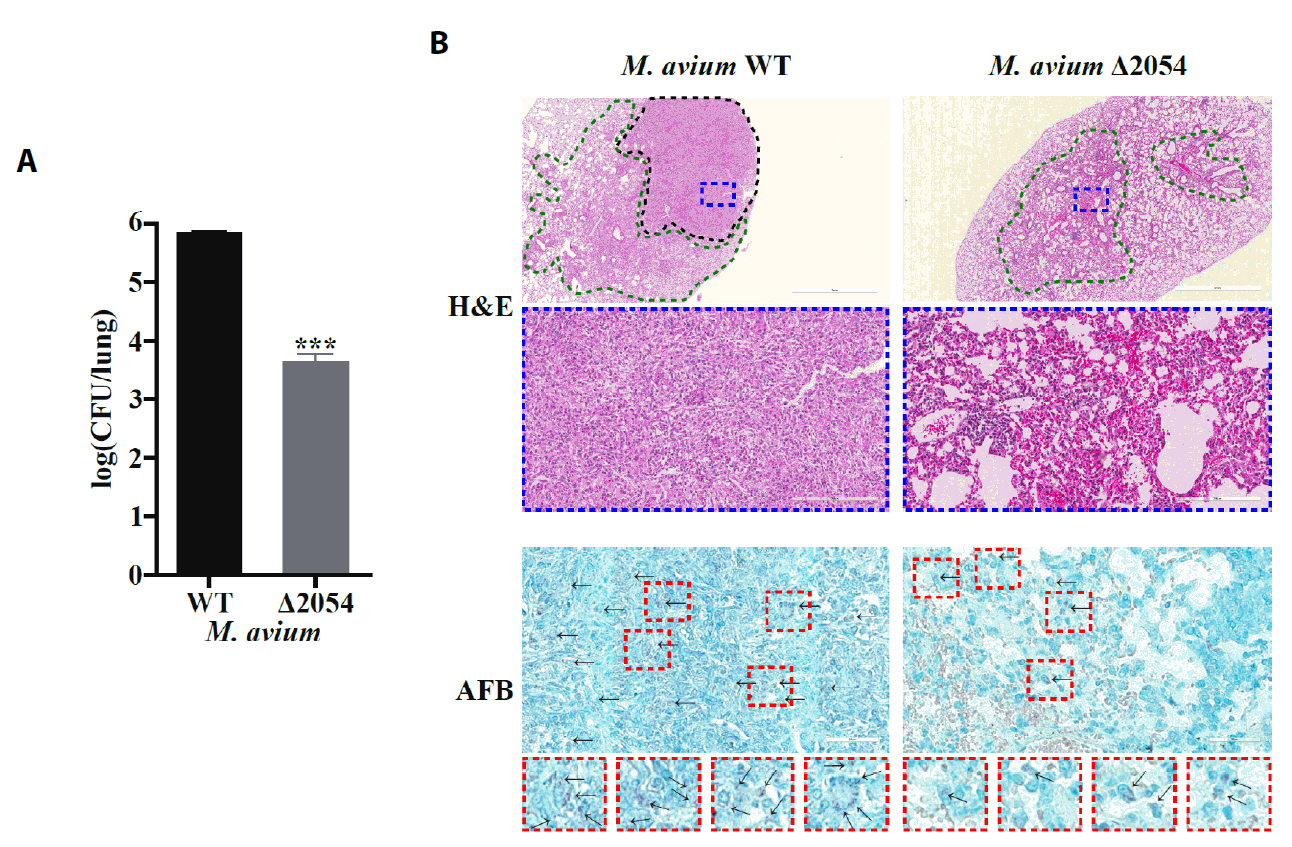
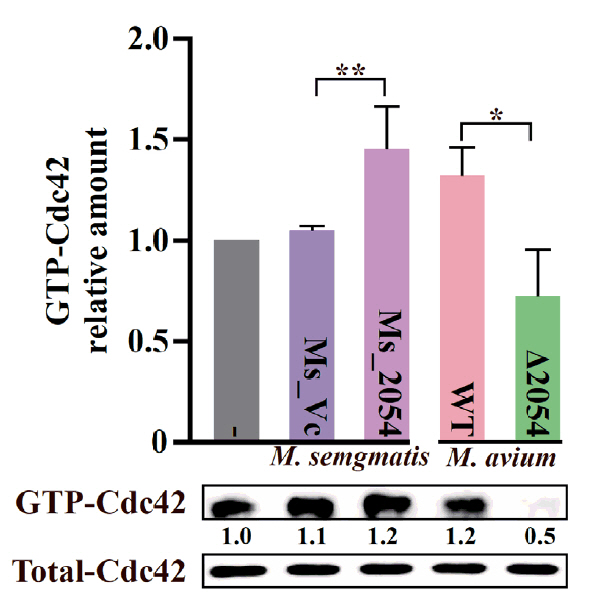
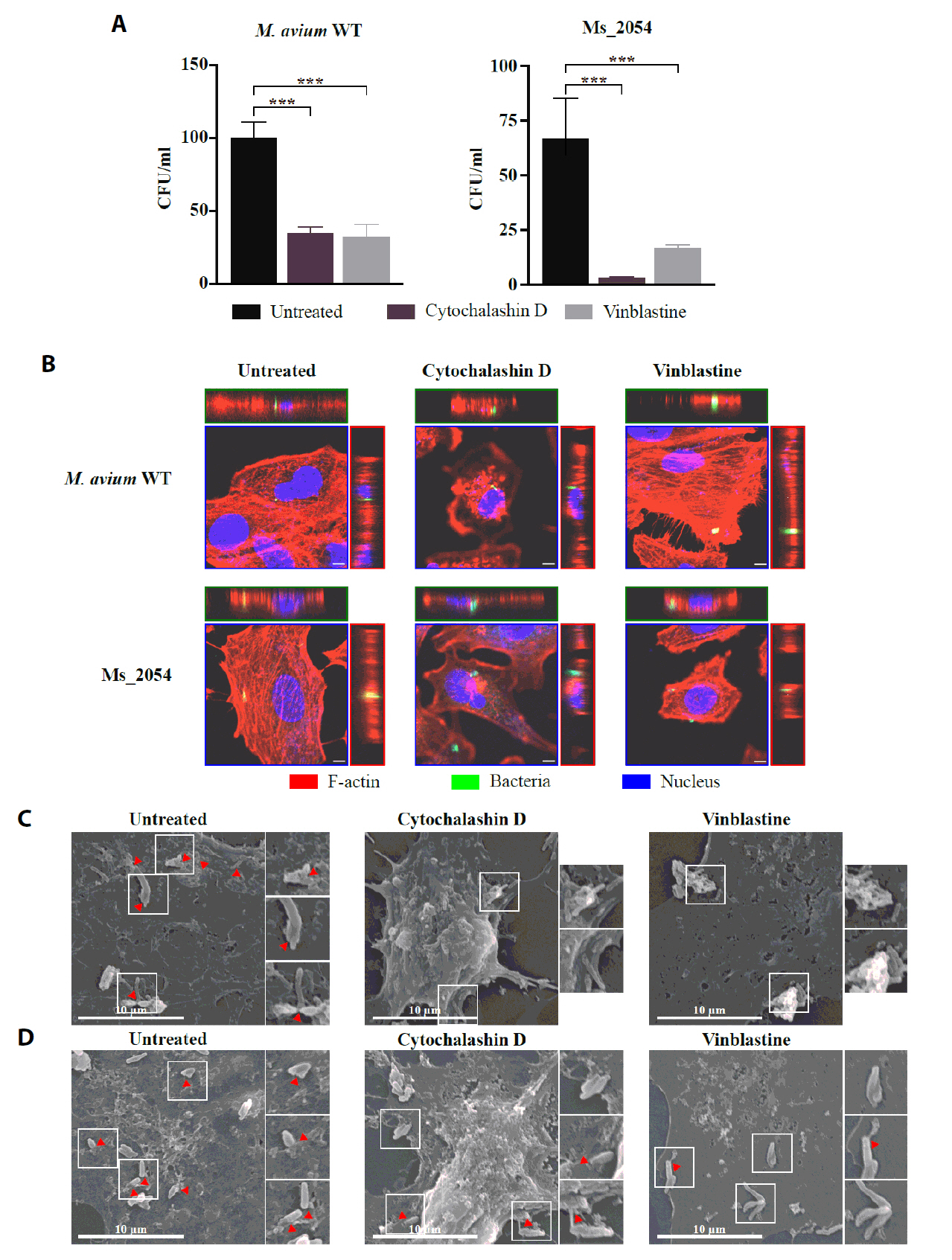
- Mycobacterium avium complex (MAC) organisms are widespread environmental pathogens associated with chronic pulmonary infections. Although M. avium is known to invade epithelial cells, the molecular mechanisms underlying this process remain incompletely understood. In this study, we identified a novel role for MAVRS09815 (formerly MAV2054), a family 2A encapsulin nanocompartment shell protein, in mediating bacterial adhesion, epithelial cell invasion, and in vivo virulence. We engineered a recombinant M. smegmatis strain expressing MAV2054 (Ms_2054) and an M. avium MAV2054 deletion mutant (Δ2054). Ms_2054 exhibited enhanced epithelial invasion, whereas Δ2054 showed reduced intracellular survival. Recombinant MAV2054 protein was bound directly to human epithelial cells in a dose-dependent manner. Pretreatment of host cells with cytochalasin D or vinblastine significantly inhibited bacterial internalization, indicating that MAV2054-mediated invasion is cytoskeleton-dependent. Confocal and scanning electron microscopy revealed MAV2054-dependent membrane rearrangements during infection. Pull-down assays demonstrated that MAV2054 activates Cdc42, a key regulator of actin polymerization, with reduced activation observed in Δ2054-infected cells. In a murine intratracheal infection model, the Δ2054 exhibited significantly reduced bacterial burdens and lung inflammation compared to the wild type. These findings demonstrate that MAV2054 enhances M. avium virulence by promoting epithelial cell invasion through Cdc42-dependent cytoskeletal remodeling. This study reveals a previously unrecognized role for an encapsulin-like protein in host-pathogen interactions and highlights its potential as a therapeutic target in MAC infections.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00273828). This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (RS-2024-00336984 and RS-2022-KH130308). This work was also supported by the NRF grant funded by the Korean government (MSIT) (RS-2024-00406568). We gratefully acknowledge Professor Hwa-Jung Kim and his laboratory for kindly providing the M. avium and M. smegmatis strains used in this study.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Statements
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Chungnam National University, Republic of Korea (approval no. 202410A-CNU-221).
- Abukhalid N, Islam S, Ndzeidze R, Bermudez LE. 2021. Mycobacterium avium subsp. hominissuis interactions with macrophage killing mechanisms. Pathogens. 10: 1365.ArticlePubMedPMC
- Andreas MP, Giessen TW. 2021. Large-scale computational discovery and analysis of virus-derived microbial nanocompartments. Nat Commun. 12: 4748.ArticlePubMedPMCPDF
- Asay BC, Edwards BB, Andrews J, Ramey ME, Richard JD, et al. 2020. Digital image analysis of heterogeneous tuberculosis pulmonary pathology in non-clinical animal models using deep convolutional neural networks. Sci Rep. 10: 6047.ArticlePubMedPMCPDF
- Bandyopadhyay S, Zhang X, Ascura A, Edelblum KL, Bonder EM, et al. 2024. Salmonella engages CDC42 effector protein 1 for intracellular invasion. J Cell Physiol. 239: 36–50. ArticlePubMed
- Bermudez LE, Shelton K, Young LS. 1995. Comparison of the ability of Mycobacterium avium, M. smegmatis and M. tuberculosis to invade and replicate within HEp-2 epithelial cells. Tuber Lung Dis. 76: 240–247. ArticlePubMed
- Bermudez LE, Wagner D, Sosnowska D. 2000. Mechanisms of Mycobacterium avium pathogenesis. Arch Immunol Ther Exp. 48: 521–527. ArticlePubMed
- Bottomley AL, Peterson E, Iosifidis G, Yong AMH, Hartley-Tassell LE, et al. 2020. The novel E. coli cell division protein, YtfB, plays a role in eukaryotic cell adhesion. Sci Rep. 10: 6745.ArticlePubMedPMCPDF
- Choi CH, Hyun SH, Lee JY, Lee JS, Lee YS, et al. 2008. Acinetobacter baumannii outer membrane protein a targets the nucleus and induces cytotoxicity. Cell Microbiol. 10: 309–319. ArticlePubMed
- Cowman S, van Ingen J, Griffith DE, Loebinger MR. 2019. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J. 54: 1900250.ArticlePubMed
- Early J, Fischer K, Bermudez LE. 2011. Mycobacterium avium uses apoptotic macrophages as tools for spreading. Microb Pathog. 50: 132–139. ArticlePubMed
- Falkinham JO 3rd. 2022. Nontuberculous mycobacteria in the environment. Tuberculosis. 137: 102267.ArticlePubMed
- Farrugia AJ, Calvo F. 2017. Cdc42 regulates Cdc42EP3 function in cancer-associated fibroblasts. Small GTPases. 8: 49–57. ArticlePubMedLink
- Fukushima S, Shimohata T, Inoue Y, Kido J, Uebanso T, et al. 2022. Recruitment of LC3 by Campylobacter jejuni to bacterial invasion site on host cells via the Rac1-mediated signaling pathway. Front Cell Infect Microbiol. 12: 829682.ArticlePubMedPMC
- Giessen TW. 2016. Encapsulins: microbial nanocompartments with applications in biomedicine, nanobiotechnology and materials science. Curr Opin Chem Biol. 34: 1–10. ArticlePubMed
- Giessen TW. 2022. Encapsulins. Annu Rev Biochem. 91: 353–380. ArticlePubMedPMC
- Huang ZW, Sutton SE, Wallenfang AJ, Orchard RC, Wu X, et al. 2009. Structural insights into host GTPase isoform selection by a family of bacterial GEF mimics. Nat Struct Mol Biol. 16: 853–860. ArticlePubMedPMCPDF
- Kim JS, Kim WS, Lee K, Won CJ, Kim JM, et al. 2013. Differential immune responses to Segniliparus rotundus and Segniliparus rugosus infection and analysis of their comparative virulence profiles. PLoS One. 8: e59646. ArticlePubMedPMC
- Krause-Gruszczynska M, Rohde M, Hartig R, Genth H, Schmidt G, et al. 2007. Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell Microbiol. 9: 2431–2444. ArticlePubMed
- Kumar K, Ponnuswamy A, Capstick TGD, Chen C, McCabe D, et al. 2024. Non-tuberculous mycobacterial pulmonary disease (NTM-PD): epidemiology, diagnosis and multidisciplinary management. Clin Med. 24: 100017.Article
- Kwon YS, Koh WJ, Daley CL. 2019. Treatment of Mycobacterium avium complex pulmonary disease. Tuberc Respir Dis. 82: 15–26. ArticleLink
- Lee K, Gallop JL, Rambani K, Kirschner MW. 2010. Self-assembly of filopodia-like structures on supported lipid bilayers. Science. 329: 1341–1345. ArticlePubMedPMC
- Lee G, Kim S, Chang S, Sohn H, Kang YA, et al. 2024. Epidemiological characteristics of nontuberculous mycobacterial pulmonary disease in South Korea: a meta-analysis of individual participant data. Tuberc Respir Dis. 87: 386–397. ArticlePDF
- Lee KI, Whang J, Choi HG, Son YJ, Jeon HS, et al. 2016. Mycobacterium avium MAV2054 protein induces macrophage apoptosis by targeting mitochondria and reduces intracellular bacterial growth. Sci Rep. 6: 37804.ArticlePubMedPMCPDF
- Lo RYC, Sorensen LS. 2007. The outer membrane protein OmpA of Mannheimia haemolytica A1 is involved in the binding of fibronectin. FEMS Microbiol Lett. 274: 226–231. ArticlePubMed
- Mahankali M, Peng HJ, Cox D, Gomez-Cambronero J. 2011. The mechanism of cell membrane ruffling relies on a phospholipase D2 (PLD2), Grb2 and Rac2 association. Cell Signal. 23: 1291–1298. ArticlePubMedPMC
- Menanteau-Ledouble S, Lawrence ML, El-Matbouli M. 2018. Invasion and replication of Yersinia ruckeri in fish cell cultures. BMC Vet Res. 14: 81.ArticlePubMedPMCPDF
- Nho JS, Jun SH, Oh MH, Park TI, Choi CW, et al. 2015. Acinetobacter nosocomialis secretes outer membrane vesicles that induce epithelial cell death and host inflammatory responses. Microb Pathog. 81: 39–45. ArticlePubMed
- Ohya K, Handa Y, Ogawa M, Suzuki M, Sasakawa C. 2005. IpgB1 is a novel Shigella effector protein involved in bacterial invasion of host cells. J Biol Chem. 280: 24022–24034. ArticlePubMed
- Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, et al. 2008. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol. 69: 164–174. ArticlePubMedPMC
- Park JH, Shin S, Kim TS, Park H. 2022. Clinically refined epidemiology of nontuberculous mycobacterial pulmonary disease in South Korea: overestimation when relying only on diagnostic codes. BMC Pulm Med. 22: 195.ArticlePubMedPMCPDF
- Recht J, Kolter R. 2001. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J Bacteriol. 183: 5718–5724. ArticlePubMedPMCLink
- Ruan C, Li J, Niu J, Li P, Huang Y, et al. 2020. Mycobacterium tuberculosis Rv0426c promotes recombinant mycobacteria intracellular survival via manipulating host inflammatory cytokines and suppressing cell apoptosis. Infect Genet Evol. 77: 104070.ArticlePubMed
- Schildkraut JA, Zweijpfenning SMH, Nap M, He K, Dacheva E, et al. 2021. The epidemiology of nontuberculous mycobacterial pulmonary disease in the Netherlands. ERJ Open Res. 7: 00207-2021.ArticlePubMedPMC
- Schorey JS, Sweet L. 2008. The mycobacterial glycopeptidolipids: structure, function, and their role in pathogenesis. Glycobiology. 18: 832–841. ArticlePubMedPMC
- Secott TE, Lin TL, Wu CC. 2004. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect Immun. 72: 3724–3732. ArticlePubMedPMCLink
- Simeone R, Bottai D, Brosch R. 2009. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr Opin Microbiol. 12: 4–10. ArticlePubMed
- Tan S, Kasperbauer S. 2021. Nontuberculous mycobacteria. Semin Respir Crit Care Med. 42: 567–586. ArticlePubMed
- To K, Cao R, Yegiazaryan A, Owens J, Venketaraman V. 2020. General overview of nontuberculous mycobacteria opportunistic pathogens: Mycobacterium avium and Mycobacterium abscessus. J Clin Med. 9: 2541.ArticlePubMedPMC
- Tsaplina O, Bozhokina E. 2021. Bacterial outer membrane protein OmpX regulates β1 integrin and epidermal growth factor receptor (EGFR) involved in invasion of M-HeLa cells by Serratia proteamaculans. Int J Mol Sci. 22: 13246.ArticlePubMedPMC
- van Ingen J, Boeree MJ, van Soolingen D, Mouton JW. 2012. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat. 15: 149–161. ArticlePubMed
- Watson JR, Owen D, Mott HR. 2017. Cdc42 in actin dynamics: an ordered pathway governed by complex equilibria and directional effector handover. Small GTPases. 8: 237–244. ArticlePubMed
- Wu ML, Aziz DB, Dartois V, Dick T. 2018. NTM drug discovery: status, gaps and the way forward. Drug Discov Today. 23: 1502–1519. ArticlePubMedPMC
- Xu T, Wang C, Li M, Yuan M, Wei J, et al. 2023. Mycobacterium tuberculosis PE8 (Rv1040c) promotes the intracellular survival of recombinant Mycobacterium by regulating host inflammatory cytokines and inhibiting cell late apoptosis. DNA Cell Biol. 42: 254–264. ArticlePubMed
- Yang W, Liu M, Yu X, Huang Y, Zeng J, et al. 2021. Mycobacterium tuberculosis Rv1515c antigen enhances survival of M. smegmatis within macrophages by disrupting the host defence. Microb Pathog. 153: 104778.ArticlePubMed
References
Figure & Data
References
Citations

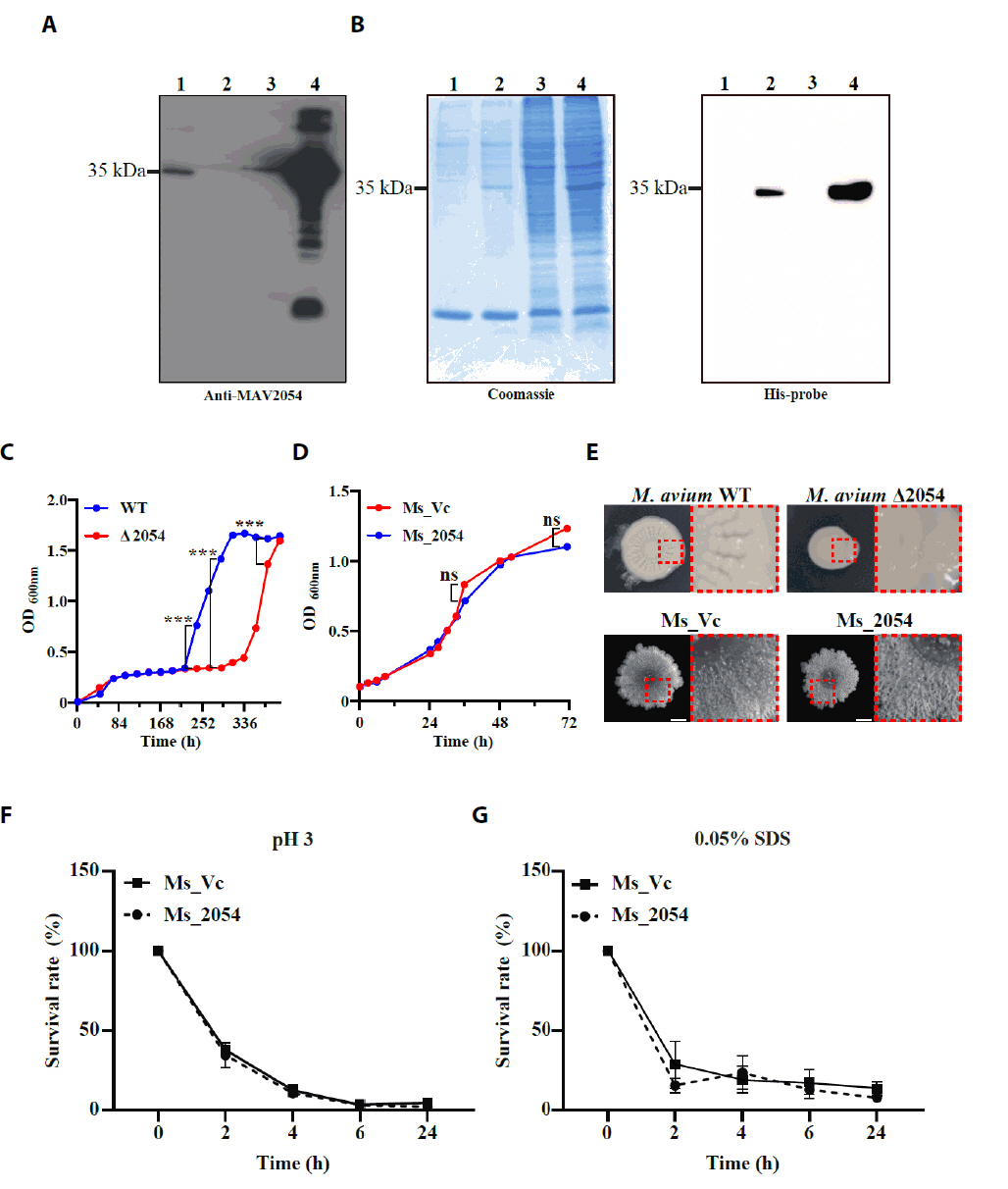
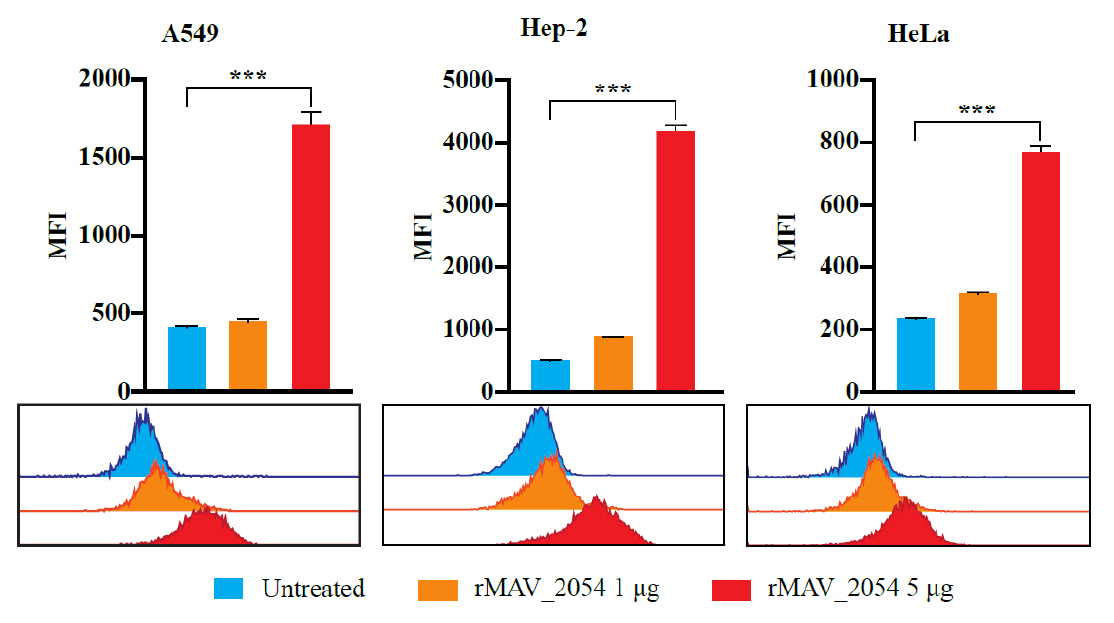
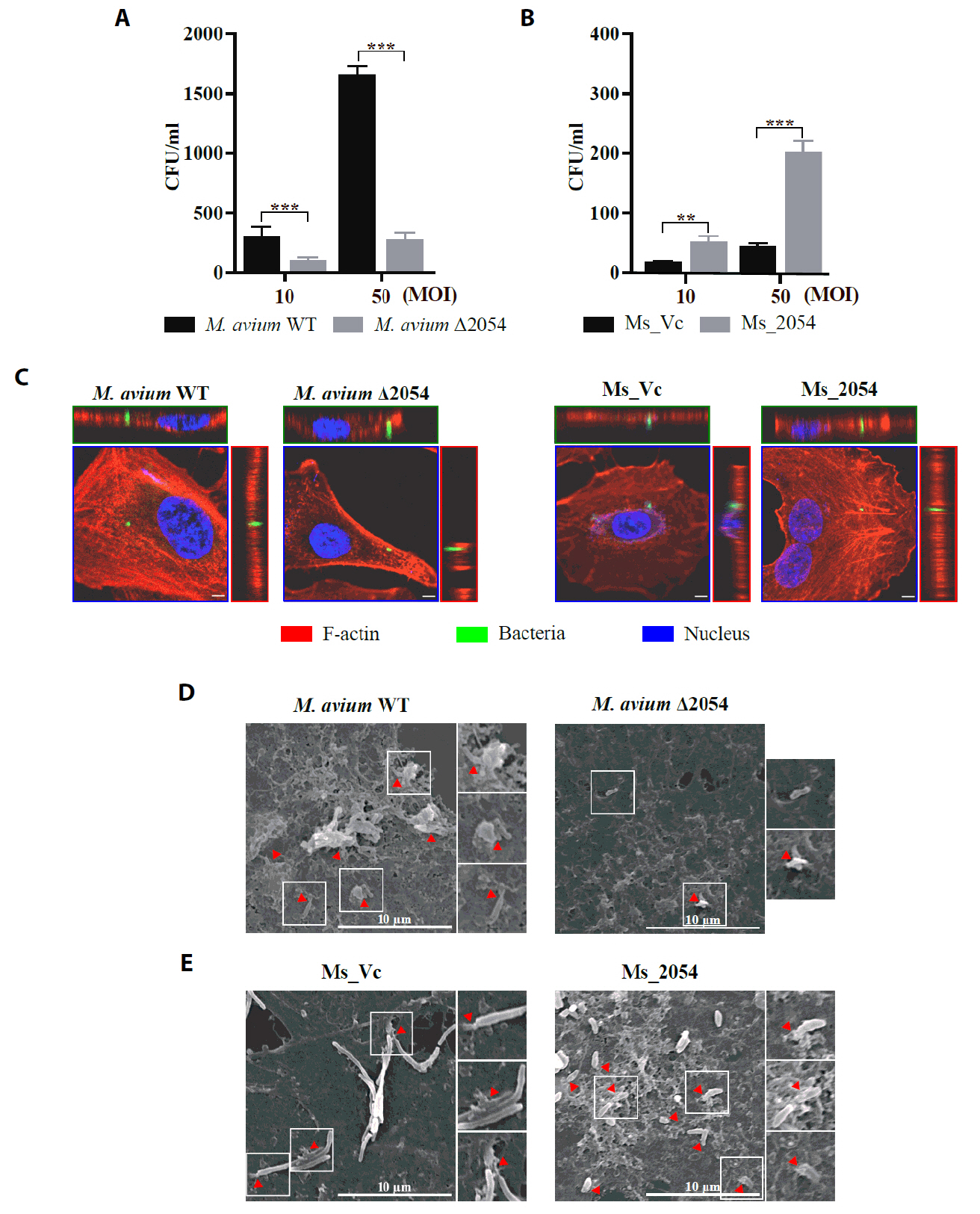
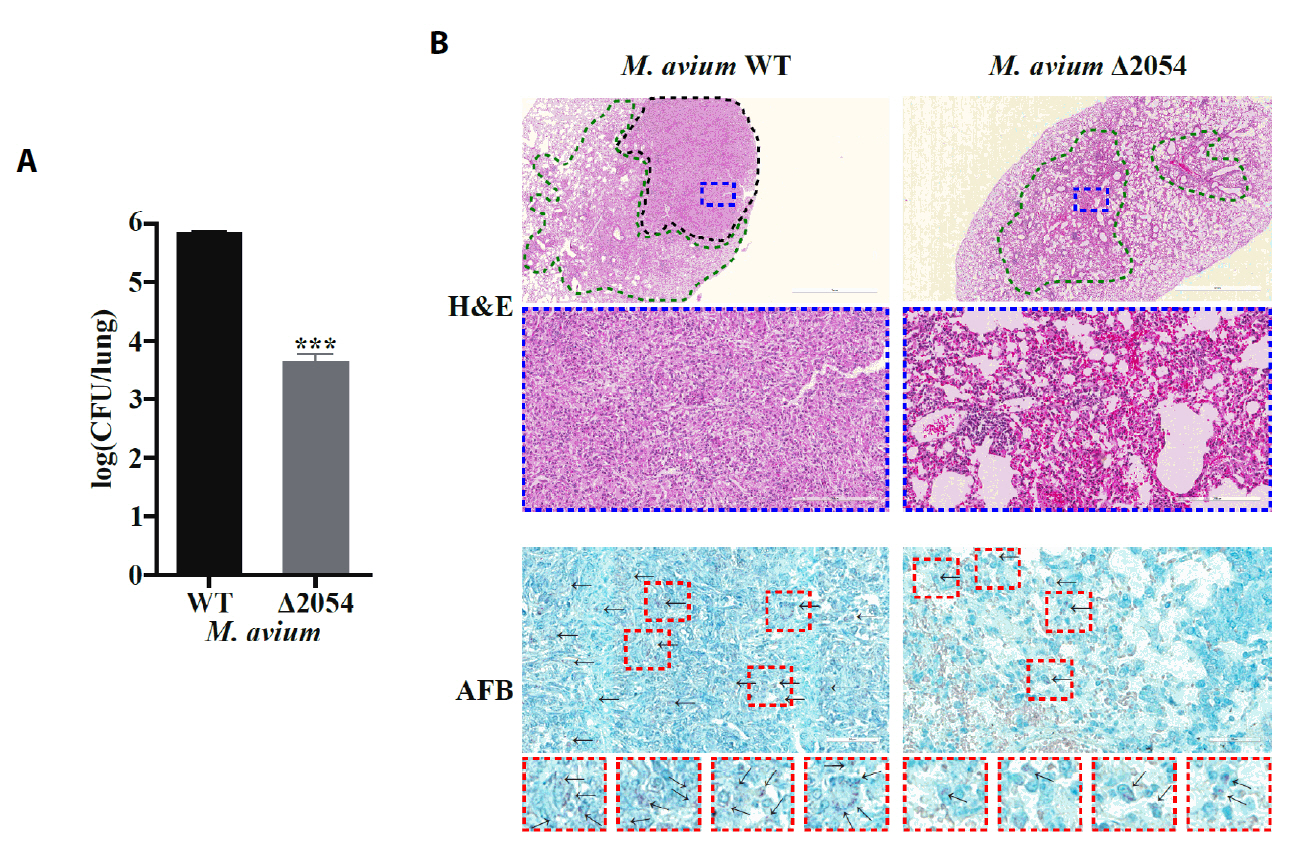
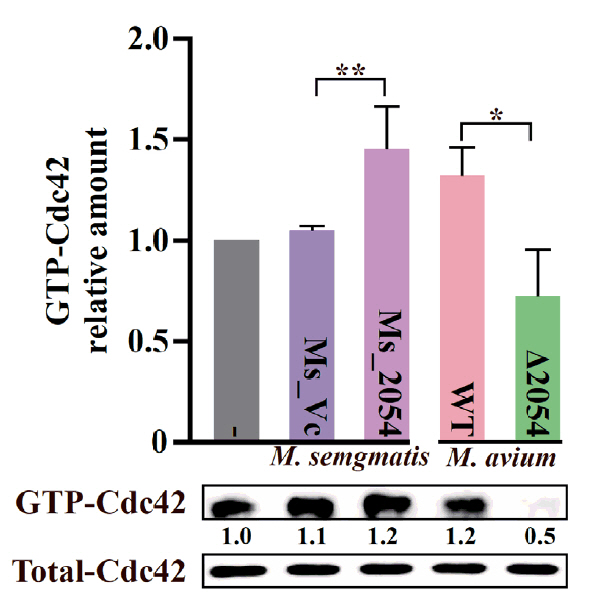
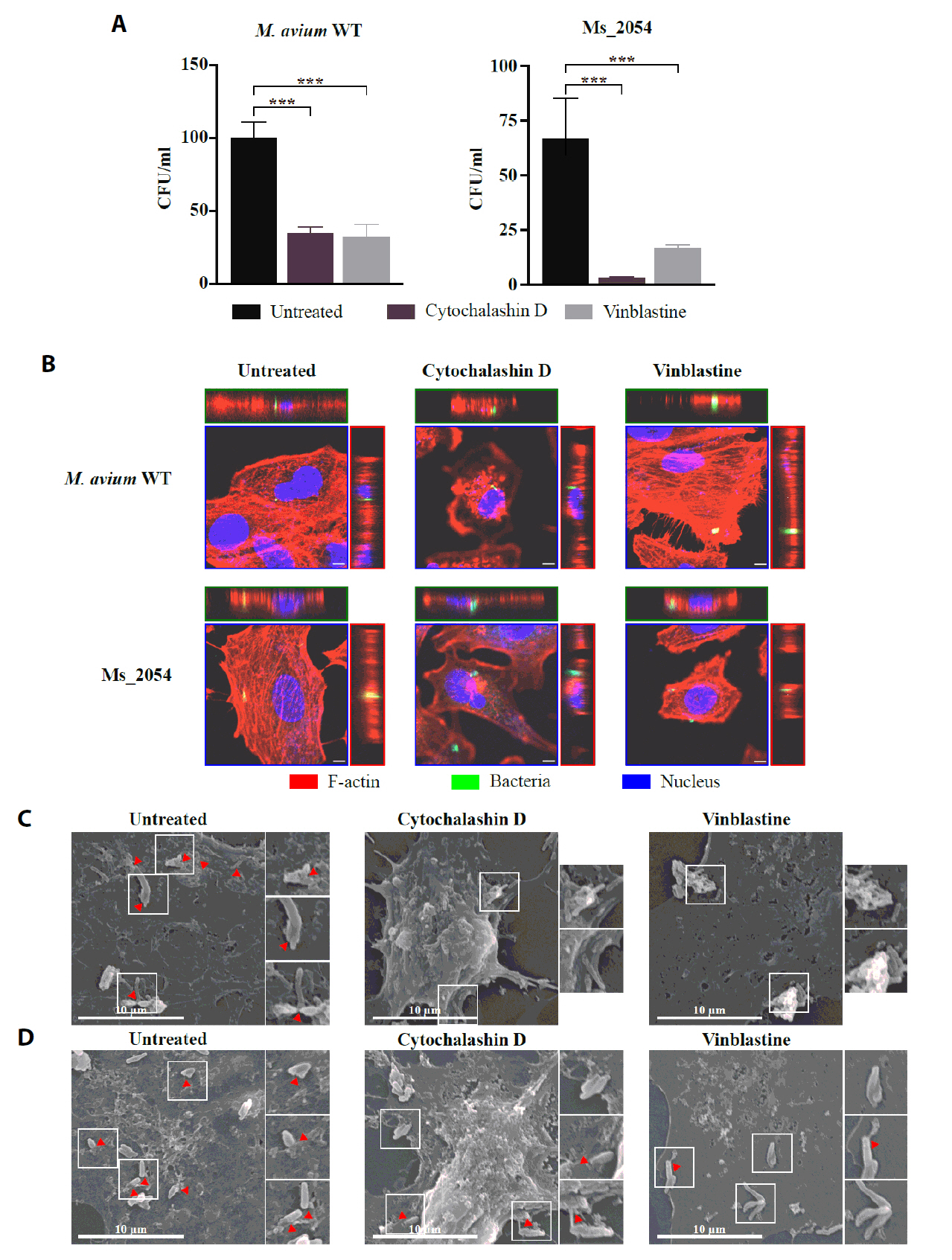
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article