Articles
- Page Path
- HOME > J. Microbiol > Volume 63(8); 2025 > Article
-
Review
CRISPR-Cas technologies: Emerging tools from research to clinical application - Hana Hyeon1,†, Soonhye Hwang1,†, Yongyang Luo1,†, Eunkyoung Shin2, Ji-Hyun Yeom1,3, Hong-Man Kim3, Minkyung Ryu1,3,*, Kangseok Lee1,*
-
Journal of Microbiology 2025;63(8):e2504012.
DOI: https://doi.org/10.71150/jm.2504012
Published online: August 31, 2025
1Department of Life Science, Chung-Ang University, Seoul 06974, Republic of Korea
2Department of Microbiology, School of Medicine, Catholic University of Daegu, Daegu 42472, Republic of Korea
3R & D Institute, NES Biotechnology, Seoul 06974, Republic of Korea
-
*For correspondence Minkyung Ryu rmk91@cau.ac.kr
Kangseok Lee kangseok@cau.ac.kr - †These authors contributed equally to this work.
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
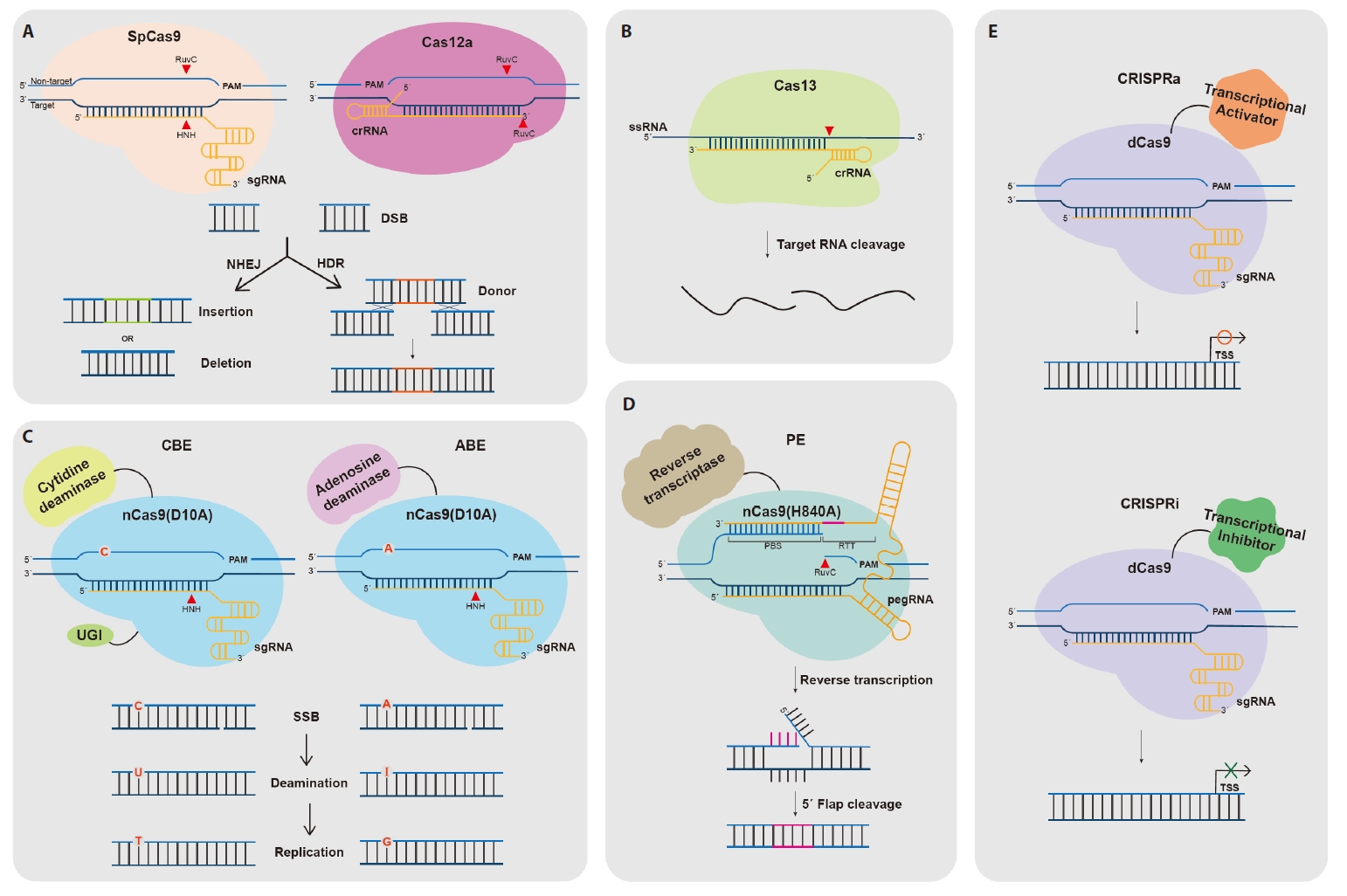
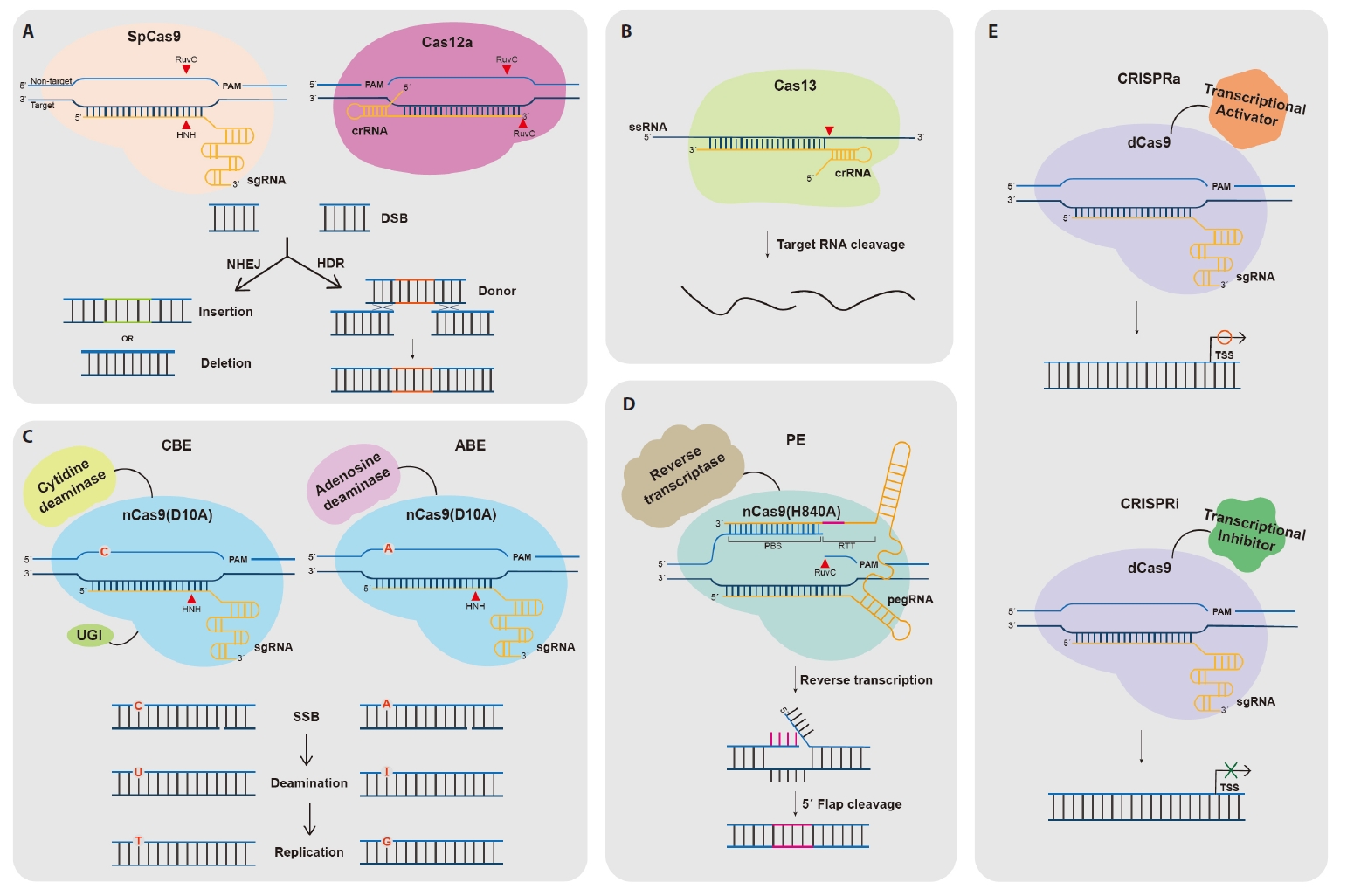
- The CRISPR-Cas Technology
- Cas Nuclease-Mediated Genome Editing
- Base Editing
- Prime Editing
- CRISPRa/i for Transcriptional Regulation
- CRISPR-Cas Delivery Formats and Tools
- CRISPR-Cas Cargo Formats
- CRISPR-Cas Delivery Systems
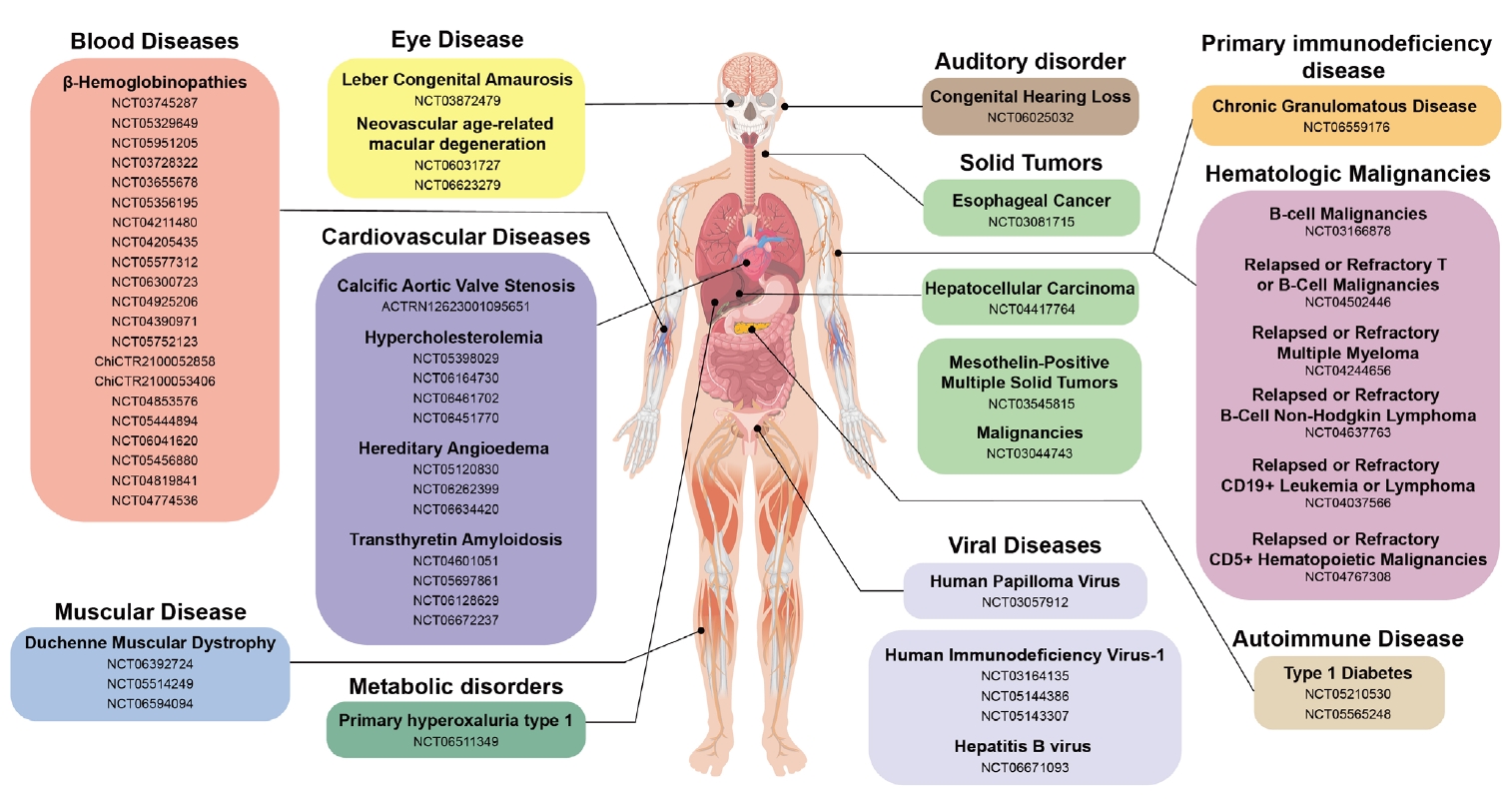
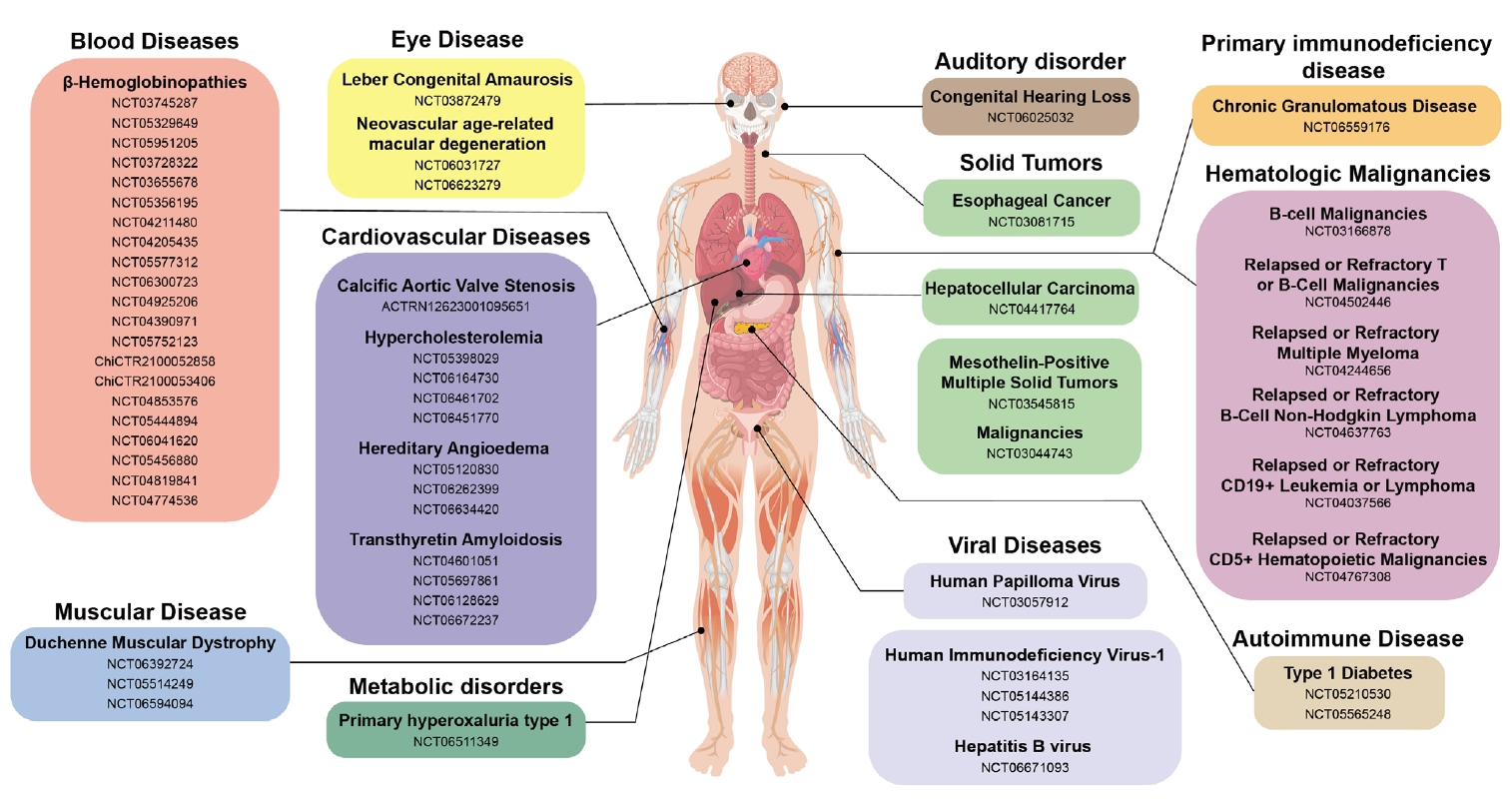
- CRISPR-Based Genetic Therapies in Clinical Trials
- Blood Disease
- Muscular Disease
- Eye Disease
- Auditory Disorder
- Autoimmune Disease: Type 1 Diabetes Mellitus (T1D)
- Metabolic Disorder
- Protein-folding Disease: Transthyretin Amyloidosis (ATTR)
- Inflammatory Disease: Hereditary Angioedema (HAE)
- Cancers
- Viral Diseases
- Primary Immunodeficiency Disease
- Concluding Remarks and Future Perspectives
- Notes
- References
ABSTRACT
- CRISPR-Cas technologies have emerged as powerful and versatile tools in gene therapy. In addition to the widely used SpCas9 system, alternative platforms including modified amino acid sequences, size-optimized variants, and other Cas enzymes from diverse bacterial species have been developed to apply this technology in various genetic contexts. In addition, base editors and prime editors for precise gene editing, the Cas13 system targeting RNA, and CRISPRa/i systems have enabled diverse and adaptable approaches for genome and RNA editing, as well as for regulating gene expression. Typically, CRISPR-Cas components are transported to the target in the form of DNA, RNA, or ribonucleoprotein complexes using various delivery methods, such as electroporation, adeno-associated viruses, and lipid nanoparticles. To amplify therapeutic efficiency, continued developments in targeted delivery technologies are required, with increased safety and stability of therapeutic biomolecules. CRISPR-based therapeutics hold an inexhaustible potential for the treatment of many diseases, including rare congenital diseases, by making permanent corrections at the genomic DNA level. In this review, we present various CRISPR-based tools, their delivery systems, and clinical progress in the CRISPR-Cas technology, highlighting its innovative prospects for gene therapy.
The CRISPR-Cas Technology
Cas Nuclease-Mediated Genome Editing
Base Editing
Prime Editing
CRISPRa/i for Transcriptional Regulation
CRISPR-Cas Delivery Formats and Tools
CRISPR-Cas Cargo Formats
CRISPR-Cas Delivery Systems
CRISPR-Based Genetic Therapies in Clinical Trials
Blood Disease
Muscular Disease
Eye Disease
Auditory Disorder
Autoimmune Disease: Type 1 Diabetes Mellitus (T1D)
Metabolic Disorder
Protein-folding Disease: Transthyretin Amyloidosis (ATTR)
Inflammatory Disease: Hereditary Angioedema (HAE)
Cancers
Viral Diseases
Primary Immunodeficiency Disease
Concluding Remarks and Future Perspectives
Acknowledgments
This research was supported by the Chung-Ang University research grant in 2022. This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (RS-2024-00461596).
Conflict of Interest
The authors have no conflict of interest.
- Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJB, Den Dunnen JT. 2006. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 34: 135–144. ArticlePubMed
- Aathira R, Jain V. 2014. Advances in management of type 1 diabetes mellitus. World J Diabetes. 5: 689–696. ArticlePubMedPMC
- Abifadel M, Rabès JP, Devillers M, Munnich A, Erlich D, et al. 2009. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat. 30: 520–529. ArticlePubMed
- Abudayyeh OO, Gootenberg JS, Essletzbichler P, Han S, Joung J, et al. 2017. RNA targeting with CRISPR-Cas13. Nature. 550: 280–284. ArticlePubMedPMCPDF
- Abudayyeh OO, Gootenberg JS, Franklin B, Koob J, Kellner MJ, et al. 2019. A cytosine deaminase for programmable single-base RNA editing. Science. 365: 382–386. ArticlePubMedPMC
- Adams D, Gonzalez-Duarte A, ORiordan WD, Yang CC, Ueda M, et al. 2018. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 379: 11–21. ArticlePubMed
- Affiliated Hospital to Academy of Military Medical Sciences. 2017. Safety of transplantation of CRISPR CCR5 modified CD34+ cells in HIV-infected subjects with hematological malignances, NCT03164135. https://clinicaltrials.gov/study/NCT03164135.
- Allife Medical Science and Technology. 2019. iHSCs with the gene correction of HBB intervent subjests with β-thalassemia mutations, NCT03728322. https://clinicaltrials.gov/study/NCT03728322.
- Alves AC, Etxebarria A, Soutar AK, Martin C, Bourbon M. 2014. Novel functional APOB mutations outside LDL-binding region causing familial hypercholesterolaemia. Hum Mol Genet. 23: 1817–1828. ArticlePubMedPDF
- Amadio M, Govoni S, Pascale A. 2016. Targeting VEGF in eye neovascularization: what's new?: a comprehensive review on current therapies and oligonucleotide-based interventions under development. Pharmacol Res. 103: 253–269. ArticlePubMed
- Amistadi S, Maule G, Ciciani M, Ensinck MM, De Keersmaecker L, et al. 2023. Functional restoration of a CFTR splicing mutation through RNA delivery of CRISPR adenine base editor. Mol Ther. 31: 1647–1660. ArticlePubMedPMC
- Amoasii L, Long C, Li H, Mireault AA, Shelton JM, et al. 2017. Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci Transl Med. 9: eaan8081. ArticlePubMedPMC
- Anders C, Niewoehner O, Duerst A, Jinek M. 2014. Structural basis of PAM-dependent target DNA recognition by the Cas9 endonuclease. Nature. 513: 569–573. ArticlePubMedPMCPDF
- Anzalone AV, Gao XD, Podracky CJ, Nelson AT, Koblan LW, et al. 2022. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat Biotechnol. 40: 731–740. ArticlePubMedPMCPDF
- Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, et al. 2019. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 576: 149–157. ArticlePubMedPMCPDF
- Aschauer DF, Kreuz S, Rumpel S. 2013. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 8: e76319. ArticlePubMedPMC
- Atkinson H, Chalmers R. 2010. Delivering the goods: viral and non-viral gene therapy systems and the inherent limits on cargo DNA and internal sequences. Genetica. 138: 485–498. ArticlePubMedPDF
- Atkinson MA, Roep BO, Posgai A, Wheeler DC, Peakman M. 2019. The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol. 7: 52–64. ArticlePubMed
- Azhar M, Phutela R, Kumar M, Ansari AH, Rauthan R, et al. 2021. Rapid and accurate nucleobase detection using FnCas9 and its application in COVID-19 diagnosis. Biosens Bioelectron. 183: 113207.ArticlePubMedPMC
- Bak RO, Dever DP, Reinisch A, Cruz Hernandez D, Majeti R, et al. 2017. Multiplexed genetic engineering of human hematopoietic stem and progenitor cells using CRISPR/Cas9 and AAV6. Elife. 6: e27873. ArticlePubMedPMCPDF
- Banno S, Nishida K, Arazoe T, Mitsunobu H, Kondo A. 2018. Deaminase-mediated multiplex genome editing in Escherichia coli. Nat Microbiol. 3: 423–429. ArticlePubMedPDF
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, et al. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 315: 1709–1712. ArticlePubMed
- Beam Therapeutics. 2022. BEACON: a study evaluating the safety and efficacy of BEAM-101 in patients with severe sickle cell disease (BEACON), NCT05456880. https://clinicaltrials.gov/study/NCT05456880.
- Behbodikhah J, Ahmed S, Elyasi A, Kasselman LJ, De Leon J, et al. 2021. Apolipoprotein B and cardiovascular disease: biomarker and potential therapeutic target. Metabolites. 11: 690.ArticlePubMedPMC
- Bioray Laboratories. 2020. Safety and efficacy evaluation of γ-globin reactivated autologous hematopoietic stem cells, NCT04211480. https://clinicaltrials.gov/study/NCT04211480.
- Bioray Laboratories. 2021. β-globin restored autologous HSC in β-thalassemia major patients, NCT04205435. https://clinicaltrials.gov/study/NCT04205435.
- Bioray Laboratories. 2022. Safety and efficacy evaluation of BRL-101 in subjects with transfusion-dependent β-thalassemia, NCT05577312. https://clinicaltrials.gov/study/NCT05577312.
- Bioray Laboratories. 2024. Clinical study of BRL-101 in severe SCD, NCT06300723. https://clinicaltrials.gov/study/NCT06300723.
- Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, et al. 2008. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 19: 1359–1368. ArticlePubMedPMC
- Bladen CL, Salgado D, Monges S, Foncuberta ME, Kekou K, et al. 2015. The TREAT-NMD DMD global database: analysis of more than 7,000 Duchenne muscular dystrophy mutations. Hum Mutat. 36: 395–402. ArticlePubMed
- Blake DJ, Weir A, Newey SE, Davies KE. 2002. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 82: 291–329. ArticlePubMed
- Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, et al. 2004. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 10: 671–678. ArticlePubMed
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 94: 1925–1930. ArticlePubMedPMC
- Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, et al. 2009. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 326: 1509–1512. ArticlePubMed
- Bressler NM, Bressler SB, Fine SL. 1988. Age-related macular degeneration. Surv Ophthalmol. 32: 375–413. ArticlePubMed
- Brooks DL, Whittaker MN, Said H, Dwivedi G, Qu P, et al. 2024. A base editing strategy using mRNA-LNPs for in vivo correction of the most frequent phenylketonuria variant. HGG Adv. 5: 100253.ArticlePubMedPMC
- Broomfield J, Hill M, Guglieri M, Crowther M, Abrams K. 2021. Life expectancy in Duchenne muscular dystrophy: reproduced individual patient data meta-analysis. Neurology. 97: e2304–e2314. ArticlePubMedPMC
- Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. 2014. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes. 7: 211–223. ArticlePubMedPMC
- Burnight ER, Gupta M, Wiley LA, Anfinson KR, Tran A, et al. 2017. Using CRISPR-Cas9 to generate gene-corrected autologous iPSCs for the treatment of inherited retinal degeneration. Mol Ther. 25: 1999–2013. ArticlePubMedPMC
- Cai Y, Cheng T, Yao Y, Li X, Ma Y, et al. 2019. In vivo genome editing rescues photoreceptor degeneration via a Cas9/RecA-mediated homology-directed repair pathway. Sci Adv. 5: eaav3335. ArticlePubMedPMC
- Canatella PJ, Karr JF, Petros JA, Prausnitz MR. 2001. Quantitative study of electroporation-mediated molecular uptake and cell viability. Biophys J. 80: 755–764. ArticlePubMedPMC
- Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, et al. 2015. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 527: 192–197. ArticlePubMedPMCPDF
- Caribou Biosciences. 2021. CRISPR-edited allogeneic anti-CD19 CAR-T cell therapy for relapsed/refractory B cell non-Hodgkin lymphoma (ANTLER), NCT04637763. https://clinicaltrials.gov/study/NCT04637763.
- Carnovale C, Bryant G, Shukla R, Bansal V. 2016. Size, shape and surface chemistry of nano-gold dictate its cellular interactions, uptake and toxicity. Prog Mater Sci. 83: 152–190. Article
- Ceccaldi R, Rondinelli B, D'Andrea AD. 2016. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 26: 52–64. ArticlePubMedPMC
- Central South University. 2019. TACE combined with PD-1 knockout engineered T cell in advanced hepatocellular carcinoma, NCT04417764. https://clinicaltrials.gov/study/NCT04417764.
- Charlesworth CT, Deshpande PS, Dever DP, Camarena J, Lemgart VT, et al. 2019. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat Med. 25: 249–254. ArticlePubMedPMCPDF
- Chavez A, Scheiman J, Vora S, Pruitt BW, Tuttle M, et al. 2015. Highly efficient Cas9-mediated transcriptional programming. Nat Methods. 12: 326–328. ArticlePubMedPMCPDF
- Chen Z, Liu F, Chen Y, Liu J, Wang X, et al. 2017. Targeted delivery of CRISPR/Cas9‐mediated cancer gene therapy via liposome‐templated hydrogel nanoparticles. Adv Funct Mater. 27: 1703036.ArticlePubMedPMCLink
- Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, et al. 2018. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 360: 436–439. ArticlePubMedPMC
- Chen S, Sun S, Moonen D, Lee C, Lee AYF, et al. 2019. CRISPR-READI: efficient generation of knockin mice by CRISPR RNP electroporation and AAV donor infection. Cell Rep. 27: 3780–3789. ArticlePubMedPMC
- Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, et al. 2020. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 15: 313–320. ArticlePubMedPMCPDF
- Chhabra A, Bashirians G, Petropoulos CJ, Wrin T, Paliwal Y, et al. 2024. Global seroprevalence of neutralizing antibodies against adeno-associated virus serotypes used for human gene therapies. Mol Ther Methods Clin Dev. 32: 101273.ArticlePubMedPMC
- Chinese PLA General Hospital. 2017. A study evaluating UCART019 in patients with relapsed or refractory CD19+ leukemia and lymphoma, NCT03166878. https://clinicaltrials.gov/study/NCT03166878.
- Chinese PLA General Hospital. 2018. Study of CRISPR-Cas9 mediated PD-1 and TCR gene-knocked out mesothelin-directed CAR-T cells in patients with mesothelin positive multiple solid tumors, NCT03545815. https://clinicaltrials.gov/study/NCT03545815.
- Cho SW, Kim S, Kim Y, Kweon J, Kim HS, et al. 2014. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 24: 132–141. ArticlePubMedPMC
- Chockalingam PS, Lin L, Chen G, Minella AC, Chen Y, et al. 2024. Impact of BEAM-101 treatment on red blood cell hemoglobin expression, rheology and sickling properties: initial data from the BEACON phase 1/2 study of autologous CD34+ base edited hematopoietic stem cells in sickle cell disease. Blood. 144: 4957.ArticlePDF
- Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, et al. 2010. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 186: 757–761. ArticlePubMedPMCPDF
- Chu SH, Ortega M, Feliciano P, Winton V, Xu C, et al. 2021. Conversion of HbS to Hb G-Makassar by adenine base editing is compatible with normal hemoglobin function. Blood. 138: 951–952. ArticleLink
- Chung JY, Ain QU, Song Y, Yong SB, Kim YH. 2019. Targeted delivery of CRISPR interference system against Fabp4 to white adipocytes ameliorates obesity, inflammation, hepatic steatosis, and insulin resistance. Genome Res. 29: 1442–1452. ArticlePubMedPMC
- Cochat P, Rumsby G. 2013. Primary hyperoxaluria. N Engl J Med. 369: 649–658. ArticlePubMed
- Cong L, Ran FA, Cox D, Lin S, Barretto R, et al. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science. 339: 819–823. ArticlePubMedPMC
- Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, et al. 2017. RNA editing with CRISPR-Cas13. Science. 358: 1019–1027. ArticlePubMedPMC
- CRISPR Therapeutics. 2020a. A safety and efficacy study evaluating CTX120 in subjects with relapsed or refractory multiple myeloma, NCT04244656. https://clinicaltrials.gov/study/NCT04244656.
- CRISPR Therapeutics. 2020b. A safety and efficacy study evaluating CTX130 in subjects with relapsed or refractory T or B cell malignancies (COBALT-LYM), NCT04502446. https://clinicaltrials.gov/study/NCT04502446.
- CRISPR Therapeutics. 2022. An open-label, FIH study evaluating the safety and tolerability of VCTX210A combination product in subjects with T1D, NCT05210530. https://clinicaltrials.gov/study/NCT05210530.
- CRISPR Therapeutics. 2023a. An open-label, FIH study evaluating the safety, tolerability, and efficacy of VCTX211 combination product in subjects with T1D, NCT05565248. https://clinicaltrials.gov/study/NCT05565248.
- CRISPR Therapeutics. 2023b. A safety and tolerability study evaluating CTX320 in subjects with elevated lipoprotein(a) and a history of atherosclerotic cardiovascular disease or calcific aortic valve stenosis. ACTRN12623001095651p. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12623001095651.
- Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, et al. 2007. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol. 14: 413–419. ArticlePubMedPDF
- Cure Rare Disease. 2022. Treatment of a single patient with CRD-TMH-001, NCT05514249. https://clinicaltrials.gov/study/NCT05514249.
- Cutler JI, Auyeung E, Mirkin CA. 2012. Spherical nucleic acids. J Am Chem Soc. 134: 1376–1391. ArticlePubMed
- Dash PK, Kaminski R, Bella R, Su H, Mathews S, et al. 2019. Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat Commun. 10: 2753.ArticlePubMedPMCPDF
- De Dreuzy E, Heath J, Zuris JA, Sousa P, Viswanathan R, et al. 2019. EDIT-301: an experimental autologous cell therapy comprising Cas12a-RNP modified mPB-CD34+ cells for the potential treatment of SCD. Blood. 134: 4636.ArticleLink
- Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, et al. 2017. Familial hypercholesterolaemia. Nat Rev Dis Primers. 3: 17093.ArticlePubMedPDF
- den Hollander AI, Koenekoop RK, Yzer S, Lopez I, Arends ML, et al. 2006. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am J Hum Genet. 79: 556–561. ArticlePubMedPMC
- Dever DP, Bak RO, Reinisch A, Camarena J, Washington G, et al. 2016. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature. 539: 384–389. ArticlePubMedPMCPDF
- DeWitt MA, Magis W, Bray NL, Wang T, Berman JR, et al. 2016. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med. 8: 360ra134.ArticlePubMedPMC
- Dong JY, Fan PD, Frizzell RA. 1996. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 7: 2101–2112. ArticlePubMed
- Dong W, Kantor B. 2021. Lentiviral vectors for delivery of gene-editing systems based on CRISPR/Cas: current state and perspectives. Viruses. 13: 1288.ArticlePubMedPMC
- Dong D, Ren K, Qiu X, Zheng J, Guo M, et al. 2016. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature. 532: 522–526. ArticlePubMedPDF
- EdiGene. 2021. A safety and efficacy study evaluating ET-01 in subjects with transfusion dependent β-thalassaemia (ET-01), NCT04925206. https://clinicaltrials.gov/study/NCT04925206.
- EdiGene. 2023. A study to evaluate the safety and efficacy of ET-01 transplantation in subjects with transfusion dependent β-thalassaemia, NCT05752123. https://clinicaltrials.gov/study/NCT05752123.
- Editas Medicine. 2019. Single ascending dose study in participants with LCA10, NCT03872479. https://clinicaltrials.gov/study/NCT03872479.
- Editas Medicine. 2021. A study evaluating the safety and efficacy of EDIT-301 in participants with severe sickle cell disease (RUBY), NCT04853576. https://clinicaltrials.gov/study/NCT04853576.
- Editas Medicine. 2022. EDIT-301 for autologous hematopoietic stem cell transplant (HSCT) in participants with transfusion-dependent beta thalassemia (TDT), NCT05444894. https://clinicaltrials.gov/study/NCT05444894.
- Excision BioTherapeutics. 2022. Study of EBT-101 in aviremic HIV-1 infected adults on stable ART, NCT05144386. https://clinicaltrials.gov/study/NCT05144386.
- Excision BioTherapeutics. 2023. Long-term follow-up study of HIV-1 infected adults who received EBT-101, NCT05143307. https://clinicaltrials.gov/study/NCT05143307.
- Fajrial AK, He QQ, Wirusanti NI, Slansky JE, Ding X. 2020. A review of emerging physical transfection methods for CRISPR/Cas9-mediated gene editing. Theranostics. 10: 5532–5549. ArticlePubMedPMC
- Fang R, Yuan P, Yu L, Yang H, Liu J, et al. 2019. Manufacturing scale-up and preclinical development of ET-01, autologous CD34+ cells with the BCL11A erythroid enhancer edited by CRISPR/Cas9, for patients with β-thalassemia major. Blood. 134: 965.ArticleLink
- Ferris FL, Fine SL, Hyman L. 1984. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 102: 1640–1642. ArticlePubMed
- Février M, Dorgham K, Rebollo A. 2011. CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses. 3: 586–612. ArticlePubMedPMC
- First Affiliated Hospital. 2018. A safety and efficacy study of TALEN and CRISPR/Cas9 in the treatment of HPV-related cervical intraepithelial neoplasia I, NCT03057912. https://clinicaltrials.gov/study/NCT03057912.
- Flotte TR, Cataltepe O, Puri A, Batista AR, Moser R, et al. 2022. AAV gene therapy for Tay-Sachs disease. Nat Med. 28: 251–259. ArticlePubMedPMC
- Fonfara I, Le Rhun A, Chylinski K, Makarova KS, Lecrivain AL, et al. 2014. Phylogeny of Cas9 determines functional exchangeability of dual-RNA and Cas9 among orthologous type II CRISPR-Cas systems. Nucleic Acids Res. 42: 2577–2590. ArticlePubMedPMCPDF
- Forget BG. 1998. Molecular basis of hereditary persistence of fetal hemoglobin. Ann N Y Acad Sci. 850: 38–44. ArticlePubMed
- Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, et al. 2021. CRISPR-Cas9 gene editing for sickle cell disease and beta-thalassemia. N Engl J Med. 384: 252–260. ArticlePubMed
- Freije CA, Myhrvold C, Boehm CK, Lin AE, Welch NL, et al. 2019. Programmable inhibition and detection of RNA viruses using Cas13. Mol Cell. 76: 826–837. ArticlePubMedPMC
- Fu B, Liao J, Chen S, Li W, Wang Q, et al. 2022. CRISPR-Cas9-mediated gene editing of the BCL11A enhancer for pediatric beta(0)/beta(0) transfusion-dependent beta-thalassemia. Nat Med. 28: 1573–1580. ArticlePubMedPDF
- Gao X, Huang L. 1996. Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry. 35: 1027–1036. ArticlePubMed
- Gao N, Zhang C, Hu Z, Li M, Wei J, et al. 2020. Characterization of Brevibacillus laterosporus Cas9 (BlatCas9) for mammalian genome editing. Front Cell Dev Biol. 8: 583164.ArticlePubMedPMC
- Gapinske M, Luu A, Winter J, Woods WS, Kostan KA, et al. 2018. CRISPR-SKIP: programmable gene splicing with single base editors. Genome Biol. 19: 107.ArticlePubMedPMCPDF
- Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, et al. 2010. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 468: 67–71. ArticlePubMedPDF
- Gasiunas G, Barrangou R, Horvath P, Siksnys V. 2012. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci USA. 109: E2579–E2586. ArticlePubMedPMC
- Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, et al. 2017. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature. 551: 464–471. ArticlePubMedPMCPDF
- GenAssist. 2024. A study to evaluate the safety and tolerability of GEN6050X in Duchenne muscular dystrophy. (GEN6050XIIT), NCT06392724. https://clinicaltrials.gov/study/NCT06392724.
- Giarrè M, Caldeira S, Malanchi I, Ciccolini F, Leão MJ, et al. 2001. Induction of pRb degradation by the human papillomavirus type 16 E7 protein is essential to efficiently overcome p16INK4a-imposed G1 cell cycle arrest. J Virol. 75: 4705–4712. ArticlePubMedPMCLink
- Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, et al. 2014. Genome-scale CRISPR-mediated control of gene repression and activation. Cell. 159: 647–661. ArticlePubMedPMC
- Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, et al. 2013. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 154: 442–451. ArticlePubMedPMC
- Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, et al. 2013. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 31: 638–646. ArticlePubMedPDF
- Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, et al. 2021. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 385: 493–502. ArticlePubMed
- Glass Z, Lee M, Li Y, Xu Q. 2018. Engineering the delivery system for CRISPR-based genome editing. Trends Biotechnol. 36: 173–185. ArticlePubMedPMC
- Glemzaite M, Balciunaite E, Karvelis T, Gasiunas G, Grusyte MM, et al. 2015. Targeted gene editing by transfection of in vitro reconstituted Streptococcus thermophilus Cas9 nuclease complex. RNA Biol. 12: 1–4. ArticlePubMedPMC
- Gruessner AC, Sutherland DE. 2005. Pancreas transplant outcomes for United States (US) and non‐US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin Transplant. 19: 433–455. ArticlePubMed
- Guiraud S, Aartsma-Rus A, Vieira NM, Davies KE, van Ommen GJ, et al. 2015. The pathogenesis and therapy of muscular dystrophies. Annu Rev Genomics Hum Genet. 16: 281–308. ArticlePubMed
- Gupta AO, Sharma A, Frangoul H, Dalal J, Kanter J, et al. 2024. Initial results from the BEACON clinical study: a phase 1/2 study evaluating the safety and efficacy of a single dose of autologous CD34+ base edited hematopoietic stem cells (BEAM-101) in patients with sickle cell disease with severe vaso-occlusive crises. Blood. 144: 513.ArticlePDF
- Hajian R, Balderston S, Tran T, deBoer T, Etienne J, et al. 2019. Detection of unamplified target genes via CRISPR-Cas9 immobilized on a graphene field-effect transistor. Nat Biomed Eng. 3: 427–437. ArticlePubMedPMCPDF
- Halbert CL, Allen JM, Miller AD. 2001. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 75: 6615–6624. ArticlePubMedPMCLink
- Hangzhou Cancer Hospital. 2017. PD-1 knockout engineered T cells for advanced esophageal cancer, NCT03081715. https://clinicaltrials.gov/study/NCT03081715.
- Hanna R, Frangoul H, McKinney C, Pineiro L, Mapara M, et al. 2023. AsCas12a gene editing of HBG1/2 promoters with EDIT-301 results in rapid and sustained normalization of hemoglobin and increased fetal hemoglobin in patients with severe sickle cell disease and transfusion-dependent beta-thalassemia. Blood. 142: 4996.ArticlePDF
- Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, et al. 2004. Pancreatic β-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 27: 2348–2355. ArticlePubMedPDF
- Heyworth PG, Cross AR, Curnutte JT. 2003. Chronic granulomatous disease. Curr Opin Immunol. 15: 578–584. ArticlePubMed
- Honegger A, Schilling D, Bastian S, Sponagel J, Kuryshev V, et al. 2015. Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog. 11: e1004712. ArticlePubMedPMC
- Hoofnagle JH. 1990. Chronic hepatitis B. N Engl J Med. 323: 337–339. ArticlePubMed
- Hou X, Zaks T, Langer R, Dong Y. 2021. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 6: 1078–1094. ArticlePubMedPMCPDF
- Hou Z, Zhang Y, Propson NE, Howden SE, Chu LF, et al. 2013. Efficient genome engineering in human pluripotent stem cells using Cas9 from Neisseria meningitidis. Proc Natl Acad Sci USA. 110: 15644–15649. ArticlePubMedPMC
- Hu JH, Miller SM, Geurts MH, Tang W, Chen L, et al. 2018. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature. 556: 57–63. ArticlePubMedPMCPDF
- Huazhong University of Science and Technology. 2021. Safety and efficacy of CT125A cells for treatment of relapsed/refractory CD5+ hematopoietic malignancies, NCT04767308. https://clinicaltrials.gov/study/NCT04767308.
- HuidaGene Therapeutics. 2023a. CRISPR/Cas13-mediated RNA targeting therapy for the treatment of neovascular age-related macular degeneration (SIGHT-I), NCT06031727. https://clinicaltrials.gov/study/NCT06031727.
- HuidaGene Therapeutics. 2023b. HuidaGene announces rare pediatric drug designation granted to HG302, a novel CRISPR DNA-editing therapy, for the treatment of Duchenne muscular dystrophy. https://www.huidagene.com/new/news/51.
- HuidaGene Therapeutics. 2023c. A study in subjects with otoferlin mutation-related hearing loss using RNA base-editing therapy (SOUND), NCT06025032. https://clinicaltrials.gov/study/NCT06025032.
- HuidaGene Therapeutics. 2024. An open-label, multidose dose-escalation study to understand the safety of CRISPR gene-editing therapy and its long-lasting effects in DMD patients (MUSCLE), NCT06594094. https://clinicaltrials.gov/study/NCT06594094.
- HuidaGene Therapeutics. 2025. Open-label dose-escalation study for CRISPR/Cas13 RNA targeting therapy for the treatment of neovascular age-related macular degeneration (BRIGHT), NCT06623279. https://clinicaltrials.gov/study/NCT06623279.
- Institute of Hematology & Blood Diseases Hospital. 2023a. Safety and efficacy evaluation of autologous CRISPR-Cas12b edited hematopoietic stem cells, NCT06041620. https://clinicaltrials.gov/study/NCT06041620.
- Institute of Hematology & Blood Diseases Hospital. 2023b. Safety and efficacy evaluation of ET-01 transplantation in subjects with transfusion dependent β-thalassaemia, NCT04390971. https://clinicaltrials.gov/study/NCT04390971.
- Intellia Therapeutics. 2020. Study to evaluate safety, tolerability, pharmacokinetics, and pharmacodynamics of NTLA-2001 in patients with hereditary transthyretin amyloidosis with polyneuropathy (ATTRv-PN) and patients with transthyretin amyloidosis-related cardiomyopathy (ATTR-CM), NCT04601051. https://clinicaltrials.gov/study/NCT04601051.
- Intellia Therapeutics. 2021. NTLA-2002 in adults with hereditary angioedema (HAE) (NTLA-2002), NCT05120830. https://clinicaltrials.gov/study/NCT05120830.
- Intellia Therapeutics. 2023a. Long-term follow-up (LTFU) of subjects dosed with NTLA-2001. https://clinicaltrials.gov/study/NCT05697861.
- Intellia Therapeutics. 2023b. MAGNITUDE: A phase 3 study of NTLA-2001 in participants with transthyretin amyloidosis with cardiomyopathy (ATTR-CM), NCT06128629. https://clinicaltrials.gov/study/NCT06128629.
- Intellia Therapeutics. 2024a. Long-term follow-up (LTFU) of subjects treated with NTLA-2002, NCT06262399. https://clinicaltrials.gov/study/NCT06262399.
- Intellia Therapeutics. 2024b. A phase 3 study of NTLA-2001 in ATTRv-PN, NCT06672237. https://clinicaltrials.gov/study/NCT06672237.
- Intellia Therapeutics. 2025. HAELO: A phase 3 study to evaluate NTLA-2002 in participants with hereditary angioedema (HAE), NCT06634420. https://clinicaltrials.gov/study/NCT06634420.
- Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. 1987. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 169: 5429–5433. ArticlePubMedPMCLink
- Iwasa YI, Nishio SY, Yoshimura H, Kanda Y, Kumakawa K, et al. 2013. OTOF mutation screening in Japanese severe to profound recessive hearing loss patients. BMC Med Genet. 14: 95.ArticlePubMedPMCPDF
- Jacobs J, Deschoolmeester V, Zwaenepoel K, Rolfo C, Silence K, et al. 2015. CD70: An emerging target in cancer immunotherapy. Pharmacol Ther. 155: 1–10. ArticlePubMed
- Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, et al. 2013. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 5: 209ra152.ArticlePubMedPMC
- Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. 2013. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol. 31: 233–239. ArticlePubMedPMCPDF
- Jiang F, Doudna JA. 2017. CRISPR-Cas9 structures and mechanisms. Annu Rev Biophys. 46: 505–529. ArticlePubMed
- Jin S, Zong Y, Gao Q, Zhu Z, Wang Y, et al. 2019. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science. 364: 292–295. ArticlePubMed
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, et al. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337: 816–821. ArticlePubMedPMC
- Kamau Therapeutics. 2021. Gene correction in autologous CD34+ hematopoietic stem cells (HbS to HbA) to treat severe sickle cell disease (Restore), NCT04819841. https://clinicaltrials.gov/study/NCT04819841.
- Kang H, Minder P, Park MA, Mesquitta WT, Torbett BE, et al. 2015. CCR5 disruption in induced pluripotent stem cells using CRISPR/Cas9 provides selective resistance of immune cells to CCR5-tropic HIV-1 virus. Mol Ther Nucleic Acids. 4: e268. ArticlePubMed
- Kanter J, DiPersio JF, Leavey P, Shyr DC, Thompson AA, et al. 2021. Cedar trial in progress: a first in human, phase 1/2 study of the correction of a single nucleotide mutation in autologous HSCs (GPH101) to convert HbS to HbA for treating severe SCD. Blood. 138: 1864.ArticleLink
- Kaplan AP, Joseph K. 2010. The bradykinin-forming cascade and its role in hereditary angioedema. Ann Allergy Asthma Immunol. 104: 193–204. ArticlePubMed
- Karpov DS, Sosnovtseva AO, Pylina SV, Bastrich AN, Petrova DA, et al. 2023. Challenges of CRISPR/Cas-based cell therapy for type 1 diabetes: how not to engineer a “Trojan horse”. Int J Mol Sci. 24: 17320.ArticlePubMedPMC
- Karvelis T, Gasiunas G, Young J, Bigelyte G, Silanskas A, et al. 2015. Rapid characterization of CRISPR-Cas9 protospacer adjacent motif sequence elements. Genome Biol. 16: 253.ArticlePubMedPMCPDF
- Kasiewicz LN, Biswas S, Beach A, Ren H, Dutta C, et al. 2023. GalNAc-lipid nanoparticles enable non-LDLR dependent hepatic delivery of a CRISPR base editing therapy. Nat Commun. 14: 2776.ArticlePubMedPMCPDF
- Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, et al. 2018. Sickle cell disease. Nat Rev Dis Primers. 4: 18010.ArticlePubMed
- Kedmi R, Ben-Arie N, Peer D. 2010. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 31: 6867–6875. ArticlePubMed
- Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. 2019. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc. 14: 2986–3012. ArticlePubMedPMCPDF
- Kelly JW, Colon W, Lai Z, Lashuel HA, Mcculloch J, et al. 1997. Transthyretin quaternary and tertiary structural changes facilitate misassembly into amyloid. Adv Protein Chem. 50: 161–181. ArticlePubMed
- Kim YG, Cha J, Chandrasegaran S. 1996. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA. 93: 1156–1160. ArticlePubMedPMC
- Kim JH, Jang HH, Ryou SM, Kim S, Bae J, et al. 2010. A functionalized gold nanoparticles-assisted universal carrier for antisense DNA. Chem Commun (Camb). 46: 4151–4153. ArticlePubMed
- Kim E, Koo T, Park SW, Kim D, Kim K, et al. 2017. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni. Nat Commun. 8: 14500.ArticlePubMedPMCPDF
- Kim DY, Lee JM, Moon SB, Chin HJ, Park S, et al. 2022. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat Biotechnol. 40: 94–102. ArticlePubMedPMCPDF
- Kim JH, Yeom JH, Ko JJ, Han MS, Lee K, et al. 2011. Effective delivery of anti-miRNA DNA oligonucleotides by functionalized gold nanoparticles. J Biotechnol. 155: 287–292. ArticlePubMed
- Kleinstiver BP, Prew MS, Tsai SQ, Topkar VV, Nguyen NT, et al. 2015. Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 523: 481–485. ArticlePubMedPMCPDF
- Ko YH. 2015. EBV and human cancer. Exp Mol Med. 47: e130.ArticlePubMedPMCPDF
- Koblan LW, Doman JL, Wilson C, Levy JM, Tay T, et al. 2018. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat Biotechnol. 36: 843–846. ArticlePubMedPMCPDF
- Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. 2016. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 533: 420–424. ArticlePubMedPMCPDF
- Komor AC, Zhao KT, Packer MS, Gaudelli NM, Waterbury AL, et al. 2017. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci Adv. 3: eaao4774. ArticlePubMedPMC
- Konermann S, Lotfy P, Brideau NJ, Oki J, Shokhirev MN, et al. 2018. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell. 173: 665–676. ArticlePubMedPMC
- Kouranova E, Forbes K, Zhao G, Warren J, Bartels A, et al. 2016. CRISPRs for optimal targeting: delivery of CRISPR components as DNA, RNA, and protein into cultured cells and single-cell embryos. Hum Gene Ther. 27: 464–475. ArticlePubMedPMC
- Krentz H, Auld M, Gill M. 2004. The high cost of medical care for patients who present late (CD4< 200 cells/μL) with HIV infection. HIV Med. 5: 93–98. ArticlePubMed
- Kulkarni A, Tanga S, Karmakar A, Hota A, Maji B. 2023. CRISPR-based precision molecular diagnostics for disease detection and surveillance. ACS Appl Bio Mater. 6: 3927–3945. ArticlePubMedLink
- Kulkarni JA, Witzigmann D, Chen S, Cullis PR, van der Meel R. 2019. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc Chem Res. 52: 2435–2444. ArticlePubMed
- Kumthekar P, Ko CH, Paunesku T, Dixit K, Sonabend AM, et al. 2021. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci Transl Med. 13: eabb3945. ArticlePubMedPMC
- Kurt IC, Zhou R, Iyer S, Garcia SP, Miller BR, et al. 2021. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat Biotechnol. 39: 41–46. ArticlePubMedPMCPDF
- Kuscu C, Parlak M, Tufan T, Yang J, Szlachta K, et al. 2017. CRISPR-STOP: gene silencing through base-editing-induced nonsense mutations. Nat Methods. 14: 710–712. ArticlePubMedPDF
- Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, et al. 2007. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 357: 2576–2588. ArticlePubMed
- Latta K, Brodehl J. 1990. Primary hyperoxaluria type I. Eur J Pediatr. 149: 518–522. ArticlePubMedPDF
- Lattanzi A, Camarena J, Lahiri P, Segal H, Srifa W, et al. 2021. Development of beta-globin gene correction in human hematopoietic stem cells as a potential durable treatment for sickle cell disease. Sci Transl Med. 13: eabf2444. ArticlePubMedPMC
- Ledford H. 2020. CRISPR treatment inserted directly into the body for first time. Nature. 579: 185.ArticlePubMedPDF
- Lee K, Conboy M, Park HM, Jiang F, Kim HJ, et al. 2017a. Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat Biomed Eng. 1: 889–901. ArticlePubMedPMCPDF
- Lee CM, Cradick TJ, Bao G. 2016. The Neisseria meningitidis CRISPR-Cas9 system enables specific genome editing in mammalian cells. Mol Ther. 24: 645–654. ArticlePubMedPMC
- Lee B, Lee K, Panda S, Gonzales-Rojas R, Chong A, et al. 2018. Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours. Nat Biomed Eng. 2: 497–507. ArticlePubMedPMCPDF
- Lee RG, Mazzola AM, Braun MC, Platt C, Vafai SB, et al. 2023. Efficacy and safety of an investigational single-course CRISPR base-editing therapy targeting PCSK9 in nonhuman primate and mouse models. Circulation. 147: 242–253. ArticlePubMed
- Lee R, Mazzola A, Denizio J, Mizoguchi T, Clendaniel V, et al. 2024. An investigational in vivo base editing medicine targeting ANGPTL3, VERVE-201, achieves precise and durable liver editing in nonclinical studies. Atherosclerosis. 395: 118496.Article
- Lee B, Park J, Ryu M, Kim S, Joo M, et al. 2017b. Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci Rep. 7: 13572.ArticlePubMedPMCPDF
- Lek A, Wong B, Keeler A, Blackwood M, Ma K, et al. 2023a. Death after high-dose rAAV9 gene therapy in a patient with Duchenne’s muscular dystrophy. N Engl J Med. 389: 1203–1210. ArticlePubMedPMC
- Lek A, Wong B, Keeler A, Blackwood M, Ma K, et al. 2023b. Unexpected death of a Duchenne muscular dystrophy patient in an N-of-1 trial of rAAV9-delivered CRISPR-transactivator. MedRxiv. doi: https://doi.org/10.1101/2023.05.16.23289881. Article
- Leonhardt C, Schwake G, Stögbauer TR, Rappl S, Kuhr JT, et al. 2014. Single-cell mRNA transfection studies: delivery, kinetics and statistics by numbers. Nanomedicine. 10: 679–688. ArticlePubMed
- Levy JM, Yeh WH, Pendse N, Davis JR, Hennessey E, et al. 2020. Cytosine and adenine base editing of the brain, liver, retina, heart and skeletal muscle of mice via adeno-associated viruses. Nat Biomed Eng. 4: 97–110. ArticlePubMedPMCPDF
- Li C, Psatha N, Sova P, Gil S, Wang H, et al. 2018. Reactivation of γ-globin in adult β-YAC mice after ex vivo and in vivo hematopoietic stem cell genome editing. Blood. 131: 2915–2928. ArticlePubMedPMCPDF
- Li ZF, Wu NQ. 2022. The progression of treatment for refractory hypercholesterolemia: focus on the prospect of gene therapy. Front Genet. 13: 911429.ArticlePubMedPMC
- Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, et al. 2009. 2-year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 136: 486–495. PubMed
- Lin Y, Wagner E, Lächelt U. 2022. Non-viral delivery of the CRISPR/Cas system: DNA versus RNA versus RNP. Biomater Sci. 10: 1166–1192. ArticlePubMed
- Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, et al. 2016. Calcific aortic stenosis. Nat Rev Dis Primers. 2: 16006.ArticlePubMedPMCPDF
- Liu B, Liu J. 2017. Methods for preparing DNA-functionalized gold nanoparticles, a key reagent of bioanalytical chemistry. Anal Methods. 9: 2633–2643. Article
- Liu L, Pei DS. 2022. Insights gained from RNA editing targeted by the CRISPR-Cas13 family. Int J Mol Sci. 23: 11400.ArticlePubMedPMC
- Liu R, Wang L, Xu H, Fang J, Liu S, et al. 2024. Updated safety and efficacy results of RM-001, autologous HBG1/2 promoter-modified CD34+ hematopoietic stem and progenitor cells, in treating transfusion-dependent β-thalassemia. Blood. 144: 4960.ArticlePDF
- Liu R, Wang L, Xu H, Yin X, Liang J, et al. 2023. S272: safety and efficacy of RM-001 in patients with transfusion-dependent Β-thalassemia: early results from the ongoing of autologous HBG1/2 promoter-modified CD34+ hematopoietic stem and progenitor cells. HemaSphere. 7: e613965e. ArticlePMC
- Longhurst HJ, Lindsay K, Petersen RS, Fijen LM, Gurugama P, et al. 2024. CRISPR-Cas9 in vivo gene editing of KLKB1 for hereditary angioedema. N Engl J Med. 390: 432–441. ArticlePubMed
- Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K, Castro MG. 2007. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 7: 347–360. ArticlePubMedPMC
- Maeder ML, Stefanidakis M, Wilson CJ, Baral R, Barrera LA, et al. 2019. Development of a gene editing approach to restore vision loss in Leber congenital amaurosis type 10. Nat Med. 25: 229–233. ArticlePubMedPDF
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, et al. 2013. RNA guided human genome engineering via Cas9. Science. 339: 823–826. ArticlePubMedPMC
- Margolin JF, Friedman JR, Meyer WK, Vissing H, Thiesen HJ, et al. 1994. Kruppel associated boxes are potent transcriptional repression domains. Proc Natl Acad Sci USA. 91: 4509–4513. ArticlePubMedPMC
- Mark Walters. 2024. Transplantation of Clustered Regularly Interspaced Short Palindromic Repeats modified hematopoietic progenitor stem cells (CRISPR_SCD001) in patients with severe sickle cell disease, NCT04774536. https://clinicaltrials.gov/study/NCT04774536.
- Mehl AL, Thomson V. 1998. Newborn hearing screening: the great omission. Pediatrics. 101: E4.ArticlePDF
- Mendell JR, Connolly AM, Lehman KJ, Griffin DA, Khan SZ, et al. 2022. Testing preexisting antibodies prior to AAV gene transfer therapy: rationale, lessons and future considerations. Mol Ther Methods Clin Dev. 25: 74–83. ArticlePubMedPMC
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al Dahhak R, et al. 2012. Evidence based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 71: 304–313. ArticlePubMed
- Migliosi V, Modamio Hoybjør S, Moreno Pelayo MA, Rodríguez Ballesteros M, Villamar M, et al. 2002. Q829X, a novel mutation in the gene encoding otoferlin (OTOF), is frequently found in Spanish patients with prelingual non syndromic hearing loss. J Med Genet. 39: 502–506. ArticlePubMedPMC
- Miller SM, Wang T, Randolph PB, Arbab M, Shen MW, et al. 2020. Continuous evolution of SpCas9 variants compatible with non G PAMs. Nat Biotechnol. 38: 471–482. ArticlePubMedPMCPDF
- Miller JB, Zhang S, Kos P, Xiong H, Zhou K, et al. 2017. Non viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed Engl. 56: 1059–1063. ArticlePubMedPMCLink
- Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, et al. 2016. Generation of stem cell derived β cells from patients with type 1 diabetes. Nat Commun. 7: 11463.ArticlePubMedPMCPDF
- Miyaoka Y, Berman JR, Cooper SB, Mayerl SJ, Chan AH, et al. 2016. Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome editing. Sci Rep. 6: 23549.ArticlePubMedPMCPDF
- Mollanoori H, Rahmati Y, Hassani B, Mehr MH, Teimourian S. 2021. Promising therapeutic approaches using CRISPR/Cas9 genome editing technology in the treatment of Duchenne muscular dystrophy. Genes Dis. 8: 146–156. ArticlePubMedPMC
- Moreno AM, Fu X, Zhu J, Katrekar D, Shih YV, et al. 2018. In situ gene therapy via AAV CRISPR Cas9 mediated targeted gene regulation. Mol Ther. 26: 1818–1827. ArticlePubMedPMC
- Morrow PK, D’Souza S, Wood T, Gowda V, Lee N, et al. 2023. CTX320: an investigational in vivo CRISPR based therapy efficiently and durably reduces lipoprotein (a) levels in non human primates after a single dose. Circulation. 148: A17013.Article
- Muller M, Lee CM, Gasiunas G, Davis TH, Cradick TJ, et al. 2016. Streptococcus thermophilus CRISPR Cas9 systems enable specific editing of the human genome. Mol Ther. 24: 636–644. ArticlePubMedPMC
- Nakamura M, Gao Y, Dominguez AA, Qi LS. 2021. CRISPR technologies for precise epigenome editing. Nat Cell Biol. 23: 11–22. ArticlePubMedPDF
- Newby GA, Yen JS, Woodard KJ, Mayuranathan T, Lazzarotto CR, et al. 2021. Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature. 595: 295–300. ArticlePubMedPMC
- Nihongaki Y, Otabe T, Ueda Y, Sato M. 2019. A split CRISPR-Cpf1 platform for inducible genome editing and gene activation. Nat Chem Biol. 15: 882–888. ArticlePubMedPDF
- Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, et al. 2016. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science. 353: aaf8729.ArticlePubMed
- Nishimasu H, Ran FA, Hsu PD, Konermann S, Shehata SI, et al. 2014. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell. 156: 935–949. ArticlePubMedPMC
- Nishimasu H, Shi X, Ishiguro S, Gao L, Hirano S, et al. 2018. Engineered CRISPR Cas9 nuclease with expanded targeting space. Science. 361: 1259–1262. ArticlePubMedPMC
- Ooi KH, Liu MM, Tay JWD, Teo SY, Kaewsapsak P, et al. 2021. An engineered CRISPR-Cas12a variant and DNA-RNA hybrid guides enable robust and rapid COVID-19 testing. Nat Commun. 12: 1739.ArticlePubMedPMCPDF
- Pal A, Kundu R. 2020. Human papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Front Microbiol. 10: 3116.ArticlePubMedPMC
- Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, et al. 2013. High-throughput profiling of off target DNA cleavage reveals RNA programmed Cas9 nuclease specificity. Nat Biotechnol. 31: 839–846. ArticlePubMedPMCPDF
- Pérez-Martínez FC, Guerra J, Posadas I, Ceña V. 2011. Barriers to non viral vector mediated gene delivery in the nervous system. Pharm Res. 28: 1843–1858. ArticlePubMedPMC
- Perez-Pinera P, Kocak DD, Vockley CM, Adler AF, Kabadi AM, et al. 2013. RNA-guided gene activation by CRISPR Cas9 based transcription factors. Nat Methods. 10: 973–976. ArticlePubMedPMCPDF
- Philippidis A. 2022. First patient dosed with VCTX210, a cell therapy for type 1 diabetes: ViaCyte and CRISPR Therapeutics are evaluating an immune evasive cell replacement therapy that they developed to help patients produce their own insulin. Genet Eng Biotechnol News. 42: 10–11. Article
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, et al. 2013. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model based map and population estimates. Lancet. 381: 142–151. ArticlePubMedPMC
- Piel FB, Steinberg MH, Rees DC. 2017. Sickle cell disease. N Engl J Med. 376: 1561–1573. ArticlePubMed
- Pierce EA, Aleman TS, Jayasundera KT, Ashimatey BS, Kim K, et al. 2024. Gene editing for CEP290-associated retinal degeneration. N Engl J Med. 390: 1972–1984. ArticlePubMedPMC
- Prausnitz MR. 1999. A practical assessment of transdermal drug delivery by skin electroporation. Adv Drug Deliv Rev. 35: 61–76. ArticlePubMed
- Prime Medicine. 2024. A study of the safety and efficacy of Prime Editing (PM359) in participants with p47phox autosomal recessive chronic granulomatous disease (CGD), NCT06559176. https://clinicaltrials.gov/study/NCT06559176?term=NCT06559176&rank=1.
- Prime Medicine. 2025. Prime Medicine announces breakthrough clinical data showing rapid restoration of DHR positivity after single infusion of PM359, an investigational prime editor for chronic granulomatous disease. https://investors.primemedicine.com/news-releases/news-release-details/prime-medicine-announces-breakthrough-clinical-data-showing.
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, et al. 2013. Repurposing CRISPR as an RNA guided platform for sequence specific control of gene expression. Cell. 152: 1173–1183. ArticlePubMedPMC
- Quan J, Langelier C, Kuchta A, Batson J, Teyssier N, et al. 2019. FLASH: a next generation CRISPR diagnostic for multiplexed detection of antimicrobial resistance sequences. Nucleic Acids Res. 47: e83. ArticlePubMedPMCPDF
- Rajamannan NM, Evans FJ, Aikawa E, Grande Allen KJ, Demer LL, et al. 2011. Calcific aortic valve disease: not simply a degenerative process a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Circulation. 124: 1783.ArticlePubMedPMC
- Ran FA, Cong LX, Yan WX, Scott DA, Gootenberg JS, et al. 2015. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 520: 186–191. ArticlePubMedPMCPDF
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, et al. 2013. Genome engineering using the CRISPR Cas9 system. Nat Protoc. 8: 2281–2308. ArticlePubMedPMCPDF
- Ranzani M, Cesana D, Bartholomae CC, Sanvito F, Pala M, et al. 2013. Lentiviral vector based insertional mutagenesis identifies genes associated with liver cancer. Nat Methods. 10: 155–161. ArticlePubMedPMCPDF
- Ren J, Liu X, Fang C, Jiang S, June CH, et al. 2017. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 23: 2255–2266. ArticlePubMedPMCPDF
- Richman DD. 2000. The impact of drug resistance on the effectiveness of chemotherapy for chronic hepatitis B. Hepatology. 32: 866–867. ArticlePubMed
- Richter MF, Zhao KT, Eton E, Lapinaite A, Newby GA, et al. 2020. Phage assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat Biotechnol. 38: 883–891. ArticlePubMedPMCPDF
- Rossidis AC, Stratigis JD, Chadwick AC, Hartman HA, Ahn NJ, et al. 2018. In utero CRISPR mediated therapeutic editing of metabolic genes. Nat Med. 24: 1513–1518. ArticlePubMedPMCPDF
- Ruan GX, Barry E, Yu D, Lukason M, Cheng SH, et al. 2017. CRISPR/Cas9 mediated genome editing as a therapeutic approach for Leber congenital amaurosis 10. Mol Ther. 25: 331–341. ArticlePubMedPMC
- Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. 2019. Transthyretin amyloid cardiomyopathy: JACC state of the art review. J Am Coll Cardiol. 73: 2872–2891. ArticlePubMedPMC
- Ruelas DS, Greene WC. 2013. An integrated overview of HIV 1 latency. Cell. 155: 519–529. ArticlePubMedPMC
- Ryou SM, Kim S, Jang HH, Kim JH, Yeom JH, et al. 2010. Delivery of shRNA using gold nanoparticle-DNA oligonucleotide conjugates as a universal carrier. Biochem Biophys Res Commun. 398: 542–546. ArticlePubMed
- Ryou SM, Yeom JH, Kang HJ, Won M, Kim JS, et al. 2014. Gold nanoparticle-DNA aptamer composites as a universal carrier for in vivo delivery of biologically functional proteins. J Control Release. 196: 287–294. ArticlePubMed
- Ryu SM, Koo T, Kim K, Lim K, Baek G, et al. 2018. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat Biotechnol. 36: 536–536. ArticlePubMedPDF
- Sakuma T, Nishikawa A, Kume S, Chayama K, Yamamoto T. 2014. Multiplex genome engineering in human cells using all in one CRISPR/Cas9 vector system. Sci Rep. 4: 5400.ArticlePubMedPMCPDF
- Salvagnin U, Unkel K, Sprink T, Bundock P, Sevenier R, et al. 2023. A comparison of three different delivery methods for achieving CRISPR/Cas9 mediated genome editing in Cichorium intybus L. Front Plant Sci. 14: 1111110.ArticlePubMedPMC
- Sander JD, Joung JK. 2014. CRISPR Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 32: 347–355. ArticlePubMedPMCPDF
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. 1993. The HPV 16 E6 and E6 AP complex functions as a ubiquitin protein ligase in the ubiquitination of p53. Cell. 75: 495–505. ArticlePubMed
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 63: 1129–1136. ArticlePubMed
- Schuh RS, Poletto É, Pasqualim G, Tavares AMV, Meyer FS, et al. 2018. In vivo genome editing of mucopolysaccharidosis I mice using the CRISPR/Cas9 system. J Control Release. 288: 23–33. ArticlePubMed
- Schumann K, Lin S, Boyer E, Simeonov DR, Subramaniam M, et al. 2015. Generation of knock in primary human T cells using Cas9 ribonucleoproteins. Proc Natl Acad Sci USA. 112: 10437–10442. ArticlePubMedPMC
- Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, et al. 2013. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 13: 653–658. ArticlePubMed
- Seger RA. 2010. Chronic granulomatous disease: recent advances in pathophysiology and treatment. Neth J Med. 68: 334–340. ArticlePubMed
- Senís E, Fatouros C, Große S, Wiedtke E, Niopek D, et al. 2014. CRISPR/Cas9 mediated genome engineering: an adeno associated viral (AAV) vector toolbox. Biotechnol J. 9: 1402–1412. ArticlePubMed
- Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. 2020. B cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 34: 985–1005. ArticlePubMedPMCPDF
- Shahbazi R, Sghia Hughes G, Reid JL, Kubek S, Haworth KG, et al. 2019. Targeted homology directed repair in blood stem and progenitor cells with CRISPR nanoformulations. Nat Mater. 18: 1124–1132. ArticlePubMedPMCPDF
- She K, Liu Y, Zhao Q, Jin X, Yang Y, et al. 2023. Dual AAV split prime editor corrects the mutation and phenotype in mice with inherited retinal degeneration. Signal Transduct Target Ther. 8: 57.ArticlePubMedPMCPDF
- Shen B, Zhang J, Wu H, Wang J, Ma K, et al. 2013. Generation of gene modified mice via Cas9/RNA mediated gene targeting. Cell Res. 23: 720–723. ArticlePubMedPMCPDF
- Shi J, Fang R, Gao Z, Shi Z, Kuang Z, et al. 2022. Preliminary safety and efficacy results of edi001: an investigator initiated trial on CRISPR/Cas9 modified autologous CD34+ hematopoietic stem and progenitor cells for patients with transfusion dependent β thalassemia. Blood. 140: 10652–10653. ArticleLink
- Shi K, Xie S, Tian R, Wang S, Lu Q, et al. 2021. A CRISPR Cas autocatalysis driven feedback amplification network for supersensitive DNA diagnostics. Sci Adv. 7: eabc7802. ArticlePubMedPMC
- Shi L, Wang S, Wu W, Wang X, Yang Q, et al. 2023. Development of an RNA targeting based gene therapy product for neovascular age related macular degeneration (nAMD). Invest Ophthalmol Vis Sci. 64: 934.
- Shi L, Wu W, Yang Q, He S, Zhu M, et al. 2024. Efficient in vitro and in vivo CRISPR RNA targeting therapy of HG202 for macular degeneration. Invest Ophthalmol Vis Sci. 65: 6517.
- Shojaeian A, Mehri-Ghahfarrokhi A. 2018. An overview of the epidemiology of type 1 diabetes mellitus. Int J Metab Syndr. 2: 1–4.
- Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, et al. 2005. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 21: 10644–10654. ArticlePubMed
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, et al. 2003. Long term follow up studies confirm the stability of the latent reservoir for HIV 1 in resting CD4+ T cells. Nat Med. 9: 727–728. ArticlePubMedPDF
- Slattery SS, Diamond A, Wang H, Therrien JA, Lant JT, et al. 2018. An expanded plasmid based genetic toolbox enables Cas9 genome editing and stable maintenance of synthetic pathways in Phaeodactylum tricornutum. ACS Synth Biol. 7: 328–338. ArticlePubMed
- Sluch V, Swain D, Whipple W, Liao M, Bhoumik A, et al. 2019. CRISPR-editing of hESCs allows for production of immune evasive cells capable of differentiation to pancreatic progenitors for future type 1 diabetes therapy. Diabetologia. 62: 6–7.
- Stella S, Alcon P, Montoya G. 2017. Structure of the Cpf1 endonuclease R loop complex after target DNA cleavage. Nature. 546: 559–563. ArticlePubMedPDF
- Stone EM. 2007. Leber congenital amaurosis: a model for efficient genetic testing of heterogeneous disorders. LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 144: 791–811. ArticlePubMed
- Storhoff JJ, Elghanian R, Mucic RC, Mirkin CA, Letsinger RL. 1998. One pot colorimetric differentiation of polynucleotides with single base imperfections using gold nanoparticle probes. J Am Chem Soc. 120: 1959–1964. Article
- Swaminathan G, Thoryk EA, Cox KS, Smith JS, Wolf JJ, et al. 2016. A tetravalent sub unit dengue vaccine formulated with ionizable cationic lipid nanoparticle induces significant immune responses in rodents and non human primates. Sci Rep. 6: 34215.ArticlePubMedPMCPDF
- Taher AT, Musallam KM, Cappellini MD. 2021. Beta thalassemias. N Engl J Med. 384: 727–743. ArticlePubMed
- Tang T, Han Y, Wang Y, Huang H, Qian P. 2021. Programmable system of Cas13 mediated RNA modification and its biological and biomedical applications. Front Cell Dev Biol. 9: 677587.ArticlePubMedPMC
- Thakore PI, Kwon JB, Nelson CE, Rouse DC, Gemberling MP, et al. 2018. RNA guided transcriptional silencing in vivo with S. aureus CRISPR Cas9 repressors. Nat Commun. 9: 1674.ArticlePubMedPMCPDF
- The First Affiliated Hospital of Guangxi Medical University. 2021. CD34 positive cells edited by autologous CRISPR Cas9 (RM 001) treatment of transfusion dependent β thalassemia safety and effectiveness exploration, ChiCTR2100053406 https://www.chictr.org.cn/showprojEN.html?proj=136638.
- The 923rd Hospital of the People's Liberation Army. 2021. Safety and efficacy evaluation of RM 001 for the treatment of transfusions dependent β thalassaemia major, ChiCTR2100052858 https://www.chictr.org.cn/showprojEN.html?proj=135649.
- Tong H, Huang J, Xiao Q, He B, Dong X, et al. 2023. High fidelity Cas13 variants for targeted RNA degradation with minimal collateral effects. Nat Biotechnol. 41: 108–119. ArticlePubMedPDF
- Truong DJ, Kühner K, Kühn R, Werfel S, Engelhardt S, et al. 2015. Development of an intein mediated split-Cas9 system for gene therapy. Nucleic Acids Res. 43: 6450–6458. ArticlePubMedPMC
- Tsang SH, Sharma T. 2018. Leber congenital amaurosis. Adv Exp Med Biol. 1085: 131–137. ArticlePubMed
- Tsong TY. 1991. Electroporation of cell membranes. Biophys J. 60: 297–306. ArticlePubMedPMC
- Tsukamoto T, Sakai E, Iizuka S, Taracena Gándara M, Sakurai F, et al. 2018. Generation of the adenovirus vector mediated CRISPR/Cpf1 system and the application for primary human hepatocytes prepared from humanized mice with chimeric liver. Biol Pharm Bull. 41: 1089–1095. ArticlePubMed
- Tune Therapeutics. 2024a. AASLD 2024: Tune Therapeutics shows near complete HepB repression with Tune 401 epigenetic silencer. https://tunetx.com/aasld-2024-tune-therapeutics-shows-near-complete-hepb-repression-with-tune-401-epigenetic-silencer/.
- Tune Therapeutics. 2024b. Phase 1b, open label study of Tune 401 to assess safety, PK and PD in adults with chronic hepatitis B, NCT06671093. https://clinicaltrials.gov/study/NCT06671093.
- Uchida E, Mizuguchi H, Ishii Watabe A, Hayakawa T. 2002. Comparison of the efficiency and safety of non viral vector mediated gene transfer into a wide range of human cells. Biol Pharm Bull. 25: 891–897. ArticlePubMed
- Uddin MN, Roni MA. 2021. Challenges of storage and stability of mRNA based COVID 19 vaccines. Vaccines. 9: 1033.ArticlePubMedPMC
- Vafai S, Karsten V, Jensen C, Falzone R, Lister T, et al. 2024. Abstract 4139206: design of Heart 2: a phase 1b clinical trial of VERVE 102, an in vivo base editing medicine delivered by a GalNAc LNP and targeting PCSK9 to durably lower LDL cholesterol. Circulation. 150: A4139206.Article
- Valkama AJ, Oruetxebarria I, Lipponen EM, Leinonen HM, Käyhty P, et al. 2020. Development of large scale downstream processing for lentiviral vectors. Mol Ther Methods Clin Dev. 17: 717–730. ArticlePubMedPMC
- VandenDriessche T, Thorrez L, Acosta Sanchez A, Petrus I, Wang L, et al. 2007. Efficacy and safety of adeno associated viral vectors based on serotype 8 and 9 vs lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost. 5: 16–24. ArticlePubMed
- Vaughan EE, Dean DA. 2006. Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol Ther. 13: 422–428. ArticlePubMedPMC
- Verdera HC, Kuranda K, Mingozzi F. 2020. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol Ther. 28: 723–746. ArticlePubMedPMC
- Vertex Pharmaceuticals. 2018a. A Safety and Efficacy Study Evaluating CTX001 in Participants With Transfusion-Dependent β-Thalassemia., NCT03655678. https://clinicaltrials.gov/study/NCT03655678.
- Vertex Pharmaceuticals. 2018b. A Safety and Efficacy Study Evaluating CTX001 in Subjects With Severe Sickle Cell Disease., NCT03745287. https://clinicaltrials.gov/study/NCT03745287.
- Vertex Pharmaceuticals. 2022a. Evaluation of Safety and Efficacy of CTX001 in Pediatric Participants With Severe Sickle Cell Disease (SCD)., NCT05329649. https://clinicaltrials.gov/study/NCT05329649.
- Vertex Pharmaceuticals. 2022b. Evaluation of Safety and Efficacy of CTX001 in Pediatric Participants With Transfusion-Dependent β-Thalassemia (TDT)., NCT05356195. https://clinicaltrials.gov/study/NCT05356195.
- Vertex Pharmaceuticals. 2024. Evaluation of efficacy and safety of a single dose of exa cel in participants with severe sickle cell disease, βS/βC genotype, NCT05951205. https://clinicaltrials.gov/study/NCT05951205.
- Verve Therapeutics. 2022. A study of VERVE 101 in patients with familial hypercholesterolemia and cardiovascular disease, NCT05398029. https://clinicaltrials.gov/study/NCT05398029.
- Verve Therapeutics. 2024a. Phase 1b study of VERVE 201 in patients with refractory hyperlipidemia, NCT06451770. https://clinicaltrials.gov/study/NCT06451770.
- Verve Therapeutics. 2024b. A study of VERVE 102 in patients with familial hypercholesterolemia or premature coronary artery disease, NCT06164730. https://clinicaltrials.gov/study/NCT06164730.
- Walsh MP, Chintakuntlawar A, Robinson CM, Madisch I, Harrach B, et al. 2009. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS One. 4: e5635. ArticlePubMedPMC
- Walton RT, Christie KA, Whittaker MN, Kleinstiver BP. 2020. Unconstrained genome targeting with near PAMless engineered CRISPR Cas9 variants. Science. 368: 290–296. ArticlePubMedPMC
- Wan P, Tang S, Lin D, Lu Y, Long M, et al. 2025. Base editing gene therapy for heterozygous familial hypercholesterolemia. medRxiv. doi: https://doi.org/10.1101/2025.04.17.25325983. Article
- Wang X, Xiong E, Tian T, Cheng M, Lin W, et al. 2020a. Clustered regularly interspaced short palindromic repeats/Cas9 mediated lateral flow nucleic acid assay. ACS Nano. 14: 2497–2508. Article
- Wang L, Xu H, Liang J, Li Y, Shi L, et al. 2022. P1465: initial safety and efficacy study of RM 001, autologous HBG1/2 promoter modified CD34+ hematopoietic stem and progenitor cells, in transfusion dependent β-thalassemia. Hemasphere. 6: 1347–1348. ArticlePMC
- Wang K, Xu J, Zhang T, Xue D. 2016. Tumor infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: a meta analysis. Oncotarget. 7: 44288–44298. ArticlePubMedPMC
- Wang D, Zhang F, Gao G. 2020b. CRISPR based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 181: 136–150. ArticlePubMedPMC
- Weaver JC, Vanbever R, Vaughan TE, Prausnitz MR. 1997. Heparin alters transdermal transport associated with electroporation. Biochem Biophys Res Commun. 234: 637–640. ArticlePubMed
- Wei T, Cheng Q, Min YL, Olson EN, Siegwart DJ. 2020. Systemic nanoparticle delivery of CRISPR Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat Commun. 11: 3232.ArticlePubMedPMCPDF
- Williams EL, Acquaviva C, Amoroso A, Chevalier F, Coulter Mackie M, et al. 2009. Primary hyperoxaluria type 1: update and additional mutation analysis of the AGXT gene. Hum Mutat. 30: 910–917. ArticlePubMed
- World Health Organization. 2021. Human genome editing: recommendations. https://www.who.int/publications/i/item/9789240030381.
- Wu Y, Zeng J, Roscoe BP, Liu P, Yao Q, et al. 2019. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat Med. 25: 776–783. ArticlePubMedPMCPDF
- Xijing Hospital. 2019. CRISPR (HPK1) edited CD19 specific CAR T cells (XYF19 CAR T cells) for CD19+ leukemia or lymphoma, NCT04037566. https://clinicaltrials.gov/study/NCT04037566.
- Xu L, Park KH, Zhao L, Xu J, El Refaey M, et al. 2016. CRISPR mediated genome editing restores dystrophin expression and function in mdx mice. Mol Ther. 24: 564–569. ArticlePubMedPMC
- Xu L, Wang J, Liu Y, Xie L, Su B, et al. 2019. CRISPR edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 381: 1240–1247. ArticlePubMed
- Xue Y, Tao Y, Wang X, Wang X, Shu Y, et al. 2023. RNA base editing therapy cures hearing loss induced by OTOF gene mutation. Mol Ther. 31: 3520–3530. ArticlePubMedPMC
- Yang Y. 2017. PD 1 knockout EBV CTLs for advanced stage Epstein Barr virus (EBV) associated malignancies, NCT03044743. https://clinicaltrials.gov/study/NCT03044743.
- Yang L, Guell M, Byrne S, Yang JL, De Los Angeles A, et al. 2013a. Optimization of scarless human stem cell genome editing. Nucleic Acids Res. 41: 9049–9061. ArticlePubMedPMC
- Yang Y, Wang L, Bell P, McMenamin D, He Z, et al. 2016. A dual AAV system enables the Cas9 mediated correction of a metabolic liver disease in newborn mice. Nat Biotechnol. 34: 334–338. ArticlePubMedPMCPDF
- Yang LZ, Wang Y, Li SQ, Yao RW, Luan PF, et al. 2019. Dynamic imaging of RNA in living cells by CRISPR Cas13 systems. Mol Cell. 76: 981–997. ArticlePubMed
- Yang T, Wei X, Chai Y, Li L, Wu H. 2013b. Genetic etiology study of the non syndromic deafness in Chinese Hans by targeted next generation sequencing. Orphanet J Rare Dis. 8: 85.ArticlePubMedPMCPDF
- Yeom JH, Lee B, Kim D, Lee JK, Kim S, et al. 2016. Gold nanoparticle DNA aptamer conjugate assisted delivery of antimicrobial peptide effectively eliminates intracellular Salmonella enterica serovar Typhimurium. Biomaterials. 104: 43–51. ArticlePubMed
- Yeom JH, Ryou SM, Won M, Park M, Bae J, et al. 2013. Inhibition of xenograft tumor growth by gold nanoparticle DNA oligonucleotide conjugates assisted delivery of BAX mRNA. PLoS One. 8: e75369. ArticlePubMedPMC
- Yeom JH, Shin E, Jin H, Liu H, Luo Y, et al. 2023. Aptamer conjugated gold nanoparticles platform as the intracellular delivery of antibodies for cancer therapy. J Ind Eng Chem. 126: 480–491. Article
- Yin H, Song CQ, Dorkin JR, Zhu LJ, Li Y, et al. 2016. Therapeutic genome editing by combined viral and non viral delivery of CRISPR system components in vivo. Nat Biotechnol. 34: 328–333. ArticlePubMedPMCPDF
- YolTech Therapeutics. 2024a. Clinical exploration study of YOLT 203 in the treatment of type 1 primary hyperoxaluria (PH1), NCT06511349. https://clinicaltrials.gov/study/NCT06511349.
- YolTech Therapeutics. 2024b. Clinical exploration trial of YOLT 101 in the treatment of familial hypercholesterolemia (FH), NCT06461702. https://clinicaltrials.gov/study/NCT06461702.
- Yoshida M, Yokota E, Sakuma T, Yamatsuji T, Takigawa N, et al. 2018. Development of an integrated CRISPRi targeting ΔNp63 for treatment of squamous cell carcinoma. Oncotarget. 9: 29220–29232. ArticlePubMedPMC
- Yu X, Liang X, Xie H, Kumar S, Ravinder N, et al. 2016. Improved delivery of Cas9 protein/gRNA complexes using lipofectamine CRISPRMAX. Biotechnol Lett. 38: 919–929. ArticlePubMedPMCPDF
- Yuan J, Ma Y, Huang T, Chen Y, Peng Y, et al. 2018. Genetic modulation of RNA splicing with a CRISPR guided cytidine deaminase. Mol Cell. 72: 380–394. ArticlePubMed
- Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, et al. 2015. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 163: 759–771. ArticlePubMedPMC
- Zhang H, Kong X, Xue M, Hu J, Wang Z, et al. 2022a. An engineered xCas12i with high activity, high specificity, and broad PAM range. Protein Cell. 14: 540–545. Article
- Zhang X, Lv S, Luo Z, Hu Y, Peng X, et al. 2021. MiniCAFE, a CRISPR/Cas9-based compact and potent transcriptional activator, elicits gene expression invivo. Nucleic Acids Res. 49: 4171–4185. ArticlePubMedPMCPDF
- Zhang H, Rombouts K, Raes L, Xiong R, De Smedt SC, et al. 2020. Fluorescence-based quantification of messenger RNA and plasmid DNA decay kinetics in extracellular biological fluids and cell extracts. Adv Biosyst. 4: 2000057.ArticleLink
- Zhang N, Si J, Li G, Wang Y, Long F, et al. 2022b. P1464Decreasing HPK1 expression in CD19 CAR T cells: a novel strategy to overcome challenges of cell therapy for adult (r/r) B ALL. Hemaspere. 23: 1346–1347. Article
- Zhang L, Wang P, Feng Q, Wang N, Chen Z, et al. 2017. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 9: e441. ArticlePDF
- Zhao D, Li J, Li S, Xin X, Hu M, et al. 2021. Glycosylase base editors enable C to A and C to G base changes. Nat Biotechnol. 39: 35–40. ArticlePubMedPDF
- Zhao Z, Shi L, Zhang W, Han J, Zhang S, et al. 2018. CRISPR knock out of programmed cell death protein 1 enhances anti tumor activity of cytotoxic T lymphocytes. Oncotarget. 9: 5208.ArticlePubMedPMC
- Zheng B, Liu R, Zhang X, Fu B, Xu Y, et al. 2023. Efficacy and safety of BRL 101, CRISPR Cas9 mediated gene editing of the BCL11A enhancer in transfusion dependent β thalassemia. Blood. 142: 4995–4996. ArticlePDF
- Zhu J, Huang X, Yang Y. 2007. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 81: 3170–3180. ArticlePubMedPMCLink
- Zoulim F, Locarnini S. 2009. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 137: 1593–1608. ArticlePubMed
- Zuo E, Sun Y, Wei W, Yuan T, Ying W, et al. 2019. Cytosine base editor generates substantial off target single nucleotide variants in mouse embryos. Science. 364: 289–292. ArticlePubMedPMC
- Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, et al. 2015. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat Biotechnol. 33: 73–80. ArticlePubMedPMCPDF
References
Figure & Data
References
Citations

- CRISPR: a precise genome editing strategy for the treatment of hepatocellular carcinoma
Subhrojyoti Mukherjee, Manish Kumar
Expert Review of Anticancer Therapy.2025; : 1. CrossRef
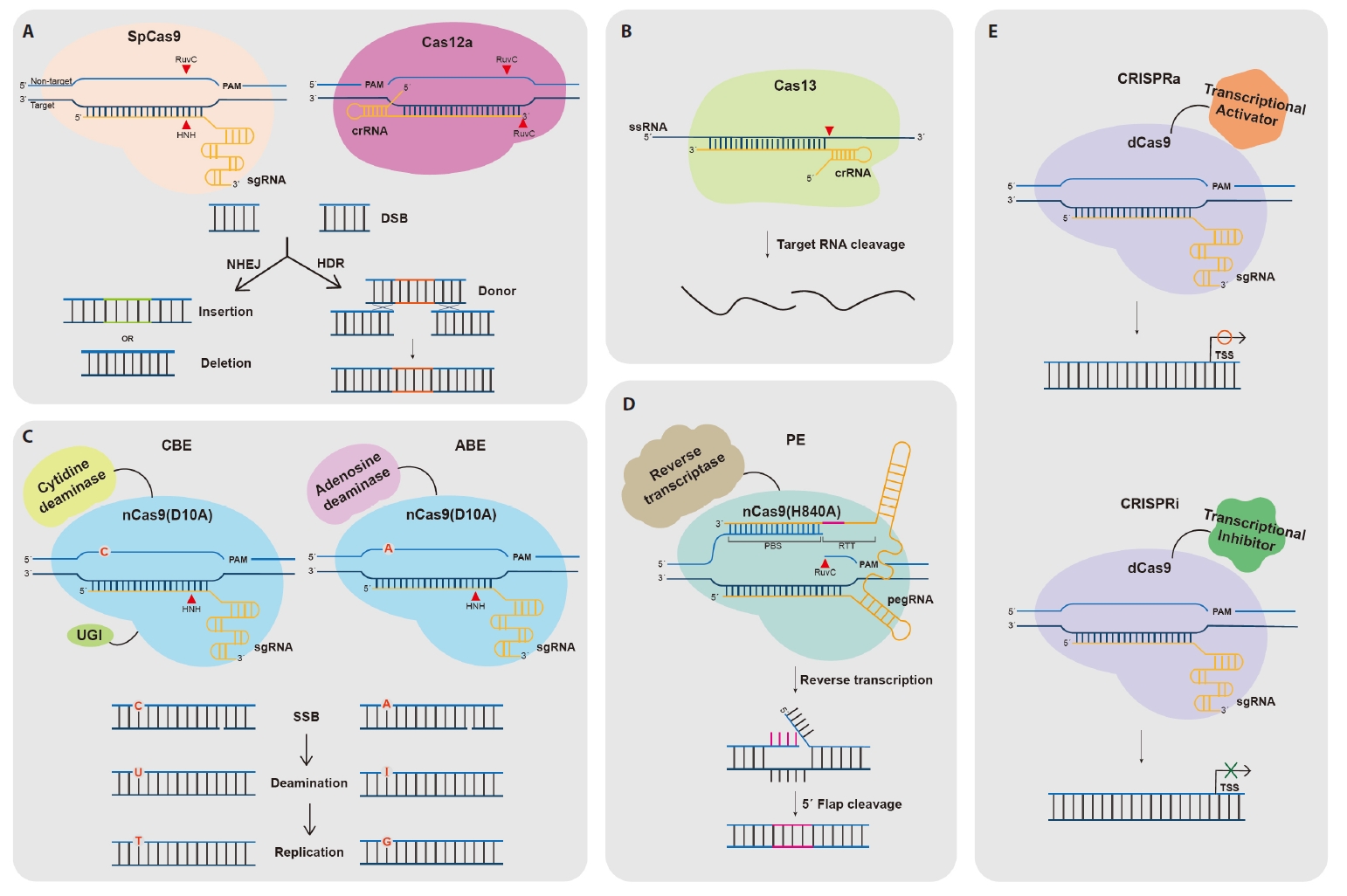
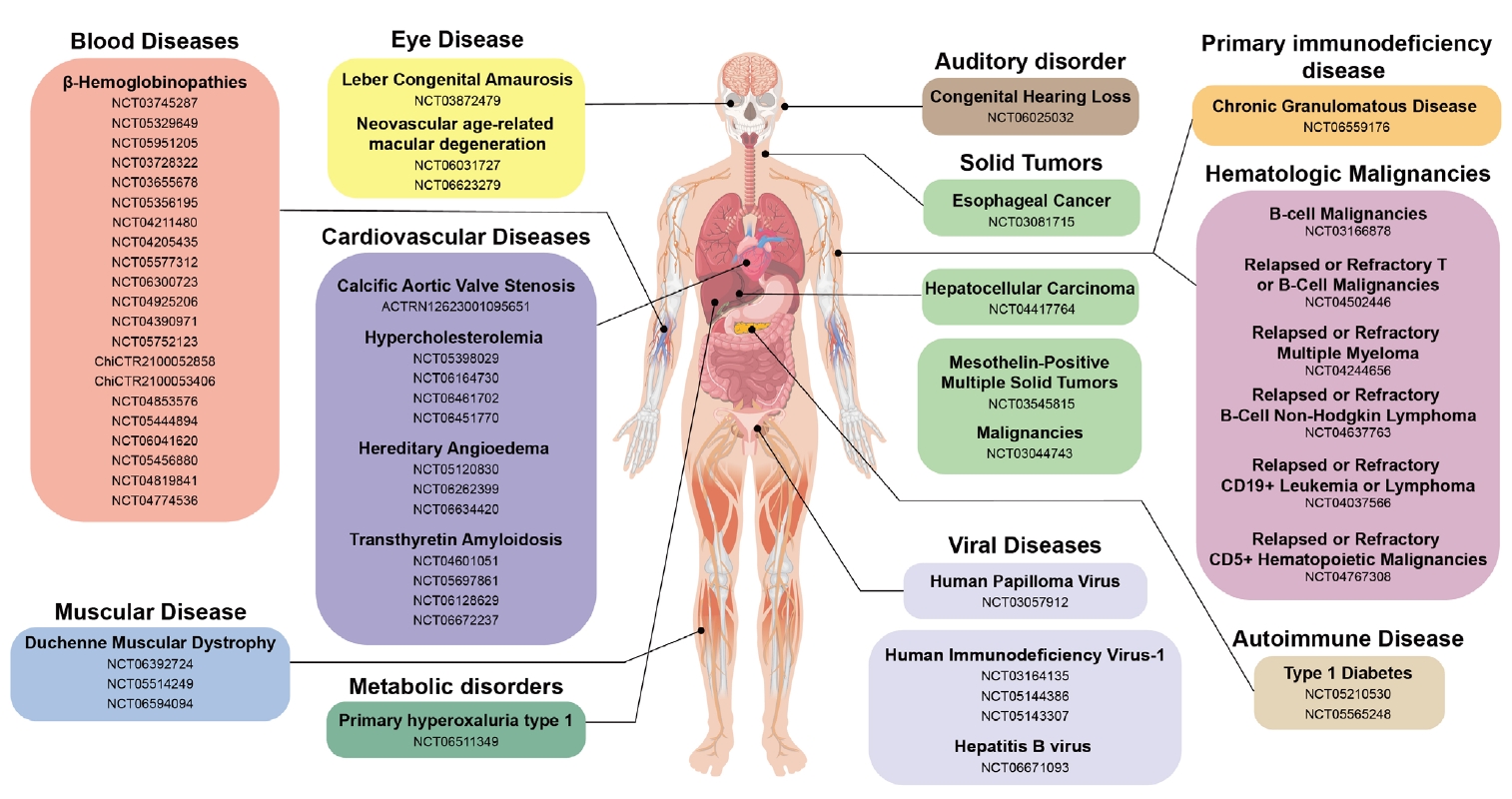
Fig. 1.
Fig. 2.
| Electroporation | AAV | LNP | AuNP | |
|---|---|---|---|---|
| CRISPR/Cas Format | DNA, mRNA, RNP | DNA | DNA, mRNA, RNP | RNP |
| Advantage | High efficiency, versatility | High efficiency, specific tissue targeting | High efficiency, versatility | High efficiency, non-toxic, specific tissue targeting |
| Disadvantage | Cell toxicity, limited applicability | Capacity limitations, high production costs, time-consuming production | Limited tissue specificity long-term safety concerns, dose-dependent toxicity | Lower efficiency, complex manufacturing |
| Disease | Target gene | Therapeutic approach | Editor | Delivery strategy | Product name | Sponsor | NCT ID | Phase |
|---|---|---|---|---|---|---|---|---|
| β-Hemoglobinopathies | ||||||||
| SCD/TDT | BCL11A enhancer | Gene disruption of the BCL11A erythroid enhancer in HSPCs via NHEJ | CRISPR-Cas9 RNP | Electroporation / ex vivo | CTX001 Exa-cel (Casgavy) | Vertex Pharmaceuticals & CRISPR Therapeutics | NCT03745287 | Approved |
| NCT05329649 | ||||||||
| NCT05951205 | ||||||||
| NCT03728322 | ||||||||
| NCT03655678 | ||||||||
| NCT05356195 | ||||||||
| TDT | BCL11A enhancer | Gene disruption of the BCL11A erythroid enhancer in HSPCs via NHEJ | CRISPR-Cas9 RNP | Electroporation / ex vivo | BRL-101 | Bioray Laboratories | NCT04211480 | Phase I |
| NCT04205435 | ||||||||
| NCT05577312 | ||||||||
| NCT06300723 | ||||||||
| TDT | BCL11A enhancer | Gene disruption of the BCL11A erythroid enhancer in HSPCs via NHEJ | CRISPR-Cas9 mRNA and sgRNA | Electroporation / ex vivo | ET-01 | EdiGene | NCT04925206 | Phase I |
| NCT04390971 | ||||||||
| NCT05752123 | ||||||||
| TDT | HBG1/2 promoter | Gene disruption of the binding sites of the HBG1/2 promoter repressor in HSPCs via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | RM-001 | Guangzhou Reforgene Medicine | ChiCTR2100052858 | Phase I |
| ChiCTR2100053406 | ||||||||
| SCD/TDT | HBG1/2 promoter | Gene disruption of the binding sites of the HBG1/2 promoter repressor in HSPCs via NHEJ | CRISPR-AsCas12a RNP | Electroporation / ex vivo | EDIT-301 (Reni-cel) | Editas Medicine | NCT04853576 | Phase I/II |
| NCT05444894 | ||||||||
| TDT | HBG1/2 promoter | Gene disruption of the binding sites of the HBG1/2 promoter repressor in HSPCs via NHEJ | CRISPR-Cas12b | Undisclosed / ex vivo | VGB-Ex01 | Shanghai Vitalgen BioPharma | NCT06041620 | N/A |
| SCD | HBG1/2 promoter | Gene disruption of the binding site of the HBG1/2 promoter repressor in HSPCs via base editing | ABE | Electroporation / ex vivo | BEAM-101 | Beam Therapeutics | NCT05456880 | Phase I/II |
| SCD | HBB | Gene correction of the β-globin locus in HSPCs via HDR | CRISPR-Cas9 RNP with DNA template (AAV6) | Electroporation / ex vivo | KMAU-001 | Kamau Therapeutics | NCT04819841 | Phase I/II |
| GPH-101 | Graphite Bio | |||||||
| Nula-Cel | ||||||||
| SCD | HBB | Gene correction of the β-globin locus in HSPCs via HDR | CRISPR-Cas9 RNP with ssODN | Electroporation / ex vivo | CRISPR_SCD001 | Mark Walters, MD | NCT04774536 | Phase I/II |
| Muscular diseases | ||||||||
| DMD | DMD | DMD exon 50 skipping via base editing | CBE | AAV9 / in vivo | GEN6050X | Peking Union Medical College Hospital | NCT06392724 | Early Phase I |
| DMD | DMD | Up-regulation of expression of the full-length isoform of dystrophin using a CRISPRa system consisting of dCas9 fused to VP64 | CRISPRa | AAV9 / in vivo | CRD-TMH-001 | Cure Rare Disease | NCT05514249 | Phase I |
| DMD | DMD | DMD exon 51 skipping and restore the correct open reading frame | CRISPR-hfCas12Max | AAV / in vivo | HG302 | HuidaGene Therapeutics | NCT06594094 | Phase I |
| Eye diseases | ||||||||
| LCA | CEP290 | Gene disruption of mutated allele in CEP290 via NHEJ | CRISPR-Cas9 | AAV5 / in vivo | EDIT-101 | Editas Medicine | NCT03872479 | Phase I/II |
| nAMD | VEGF | Knock down the expression of VEGFA | CRISPR-hfCas13Y | AAV / in vivo | HG202 | HuidaGene Therapeutics | NCT06031727 | Phase I |
| NCT06623279 | ||||||||
| Auditory disorder | ||||||||
| Congenital Hearing Loss | OTOF | RNA base editing of p.Q829X mutation in OTOF gene | CRISPR-Cas13 | AAV / in vivo | HG205 | HuidaGene Therapeutics | NCT06025032 | Early Phase I |
| Autoimmune diseases | ||||||||
| T1D | knockouts (B2M, TXNIP) insertions (PD-L1, HLA-E, TNFAIP3, and MANF) | PEC210A (allogeneic pancreatic endoderm cells) or PEC211 (allogeneic stem cell) modified using CRISPR-Cas9 | CRISPR-Cas9 | Undisclosed / ex vivo | VCTX210A | CRISPR Therapeutics & Viacyte | NCT05210530 | Phase I |
| VCTX211 | NCT05565248 | Phase I/II | ||||||
| Metabolic disorders | ||||||||
| Calcific Aortic Valve Stenosis | LPA | Gene disruption of LPA via NHEJ | CRISPR-Cas9 mRNA and sgRNA | LNP / in vivo | CTX-320 | CRISPR Therapeutics AG | ACTRN12623001095651p | Phase I |
| PH1 | HAO1 | Gene disruption of HAO1 via NHEJ | CRISPR-Cas12 | LNP / in vivo | YOLT-203 | RenJi Hospital | NCT06511349 | Early Phase I |
| Hypercholesterolemia | ||||||||
| HeFH | PCSK9 | Gene disruption of PCSK9 via base-editing | ABE mRNA and sgRNA | LNP / in vivo | VERVE-101 | Verve Therapeutics | NCT05398029 | Phase I |
| VERVE-102 | NCT06164730 | |||||||
| HeFH | PCSK9 | Gene disruption of PCSK9 via base-editing | ABE mRNA and sgRNA | LNP / in vivo | YOLT-101 | YolTech Therapeutics | NCT06461702 | Early Phase I |
| HoFH / RH | ANGPTL3 | Gene disruption of ANGPTL3 via base-editing | ABE mRNA and sgRNA | LNP / in vivo | VERVE-201 | Verve Therapeutics | NCT06451770 | Phase I |
| Protein-folding disease | ||||||||
| ATTR | TTR | Gene disruption of TTR via NHEJ | CRISPR-Cas9 mRNA and sgRNA | LNP / in vivo | NTLA-2001 | Intellia Therapeutics | NCT04601051 | Phase III |
| NCT05697861 | ||||||||
| NCT06128629 | ||||||||
| NCT06672237 | ||||||||
| Inflammatory diseases | ||||||||
| HAE | KLKB1 | Gene disruption of KLKB1 via NHEJ | CRISPR-Cas9 mRNA and sgRNA | LNP / in vivo | NTLA-2002 | Intellia Therapeutics | NCT05120830 | Phase III |
| NCT06262399 | ||||||||
| NCT06634420 | ||||||||
| Cancers | ||||||||
| Esophageal Cancer | PD-1 | Gene disruption of PD-1 in TILs via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | Hangzhou Cancer Hospital | NCT03081715 | N/A | |
| HCC | PD-1 | Gene disruption of PD-1 in TILs via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | Central South University | NCT04417764 | Phase I | |
| Malignancies | PD-1 | Gene disruption of PD-1 in EBV-specific CTLs via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | Yang Yang | NCT03044743 | Phase I/II | |
| Mesothelin-positive Multiple Solid Tumors | PD-1 and TCR | Gene disruption of PD-1 and TCR in anti-mesothelin CAR-T cells via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | Chinese PLA General Hospital | NCT03545815 | Phase I | |
| B-cell Malignancies | B2M and TCR | Gene disruption of B2M and TCR in anti-CD19 CAR-T cells via NHEJ | CRISPR-Cas9 | Electroporation / ex vivo | UCART019 | Chinese PLA General Hospital | NCT03166878 | Phase I/II |
| Relapsed or Refractory T or B-Cell Malignancies | B2M and TCR | Gene disruption of B2M and TCR in anti-CD70 CAR-T cells via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | CTX130 | CRISPR Therapeutics AG | NCT04502446 | Phase I |
| Relapsed or Refractory Multiple Myeloma | B2M and TCR | Gene disruption of B2M and TCR in anti-BCMA CAR-T cells via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | CTX120 | CRISPR Therapeutics AG | NCT04244656 | Phase I |
| Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma | PD-1 and TCR | Gene disruption of PD-1 and TCR in anti-CD19 CAR-T cells via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | CB-010 | Caribou Biosciences | NCT04637763 | Phase I |
| Relapsed or Refractory CD19+ Leukemia or Lymphoma | HPK1 | Gene disruption of HPK1 in anti-CD19 CAR-T cells via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | XYF19 | Xijing Hospital | NCT04037566 | Phase I |
| Relapsed/Refractory CD5+ Hematopoietic Malignancies | CD5 and TCR | Gene disruption of CD5 and TCR in anti-CD5 CAR-T cells via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | CT125A | Huazhong University of Science and Technology | NCT04767308 | Early Phase I |
| Viral diseases | ||||||||
| HPV | HPV E6/E7 | Gene disruption of HPV E6/E7 via NHEJ | CRISPR-Cas9 | LNP / in vivo | First Affiliated Hospital, Sun Yat-Sen University | NCT03057912 | Phase I | |
| HIV-1 | CCR5 | Gene disruption of CCR5 in allogeneic stem cell via NHEJ | CRISPR-Cas9 | Undisclosed / ex vivo | Affiliated Hospital to Academy of Military Medical Sciences | NCT03164135 | N/A | |
| HIV-1 | 5′- & 3′-LTRs and gag | Excising large portions of the HIV genome via NHEJ | CRISPR-Cas9 | AAV9 / in vivo | EBT-101 | Excision BioTherapeutics | NCT05144386 | Phase I |
| NCT05143307 | ||||||||
| HBV | HBV | Epigenetic gene silencing through DNA methylation and heterochromatin formation | Epigenetic editing | LNP / in vivo | Tune-401 | Tune Therapeutics | NCT06671093 | Phase I |
| Primary immunodeficiency disease | ||||||||
| CGD | NCF1 | Prime editing of HSPCs targeting NCF1 mutation | PE | Undisclosed / ex vivo | PM359 | Prime Medicine | NCT06559176 | Phase I/II |
Table 1.
Table 2.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article