Articles
- Page Path
- HOME > J. Microbiol > Volume 63(7); 2025 > Article
-
Review
Extracellular vesicles of Gram-negative and Gram-positive probiotics - Yangyunqi Wang1,2,†, Chongxu Duan1,2,†, Xiaomin Yu2,3,*
-
Journal of Microbiology 2025;63(7):e2506005.
DOI: https://doi.org/10.71150/jm.2506005
Published online: July 31, 2025
1Queen Mary School, Jiangxi Medical College, Nanchang University, Nanchang 330006, P. R. China
2School of Basic Medical Science, Jiangxi Medical College, Nanchang University, Nanchang 330006, P. R. China
3Medical Experimental Teaching Center, School of Basic Medical Science, Jiangxi Medical College, Nanchang University, Nanchang 330006, P. R. China
- *Correspondence Xiaomin Yu yuxiaomin@ncu.edu.cn
- †These authors contributed equally to this work.
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ABSTRACT
- Introduction
- Biological Effects of Probiotic EVs
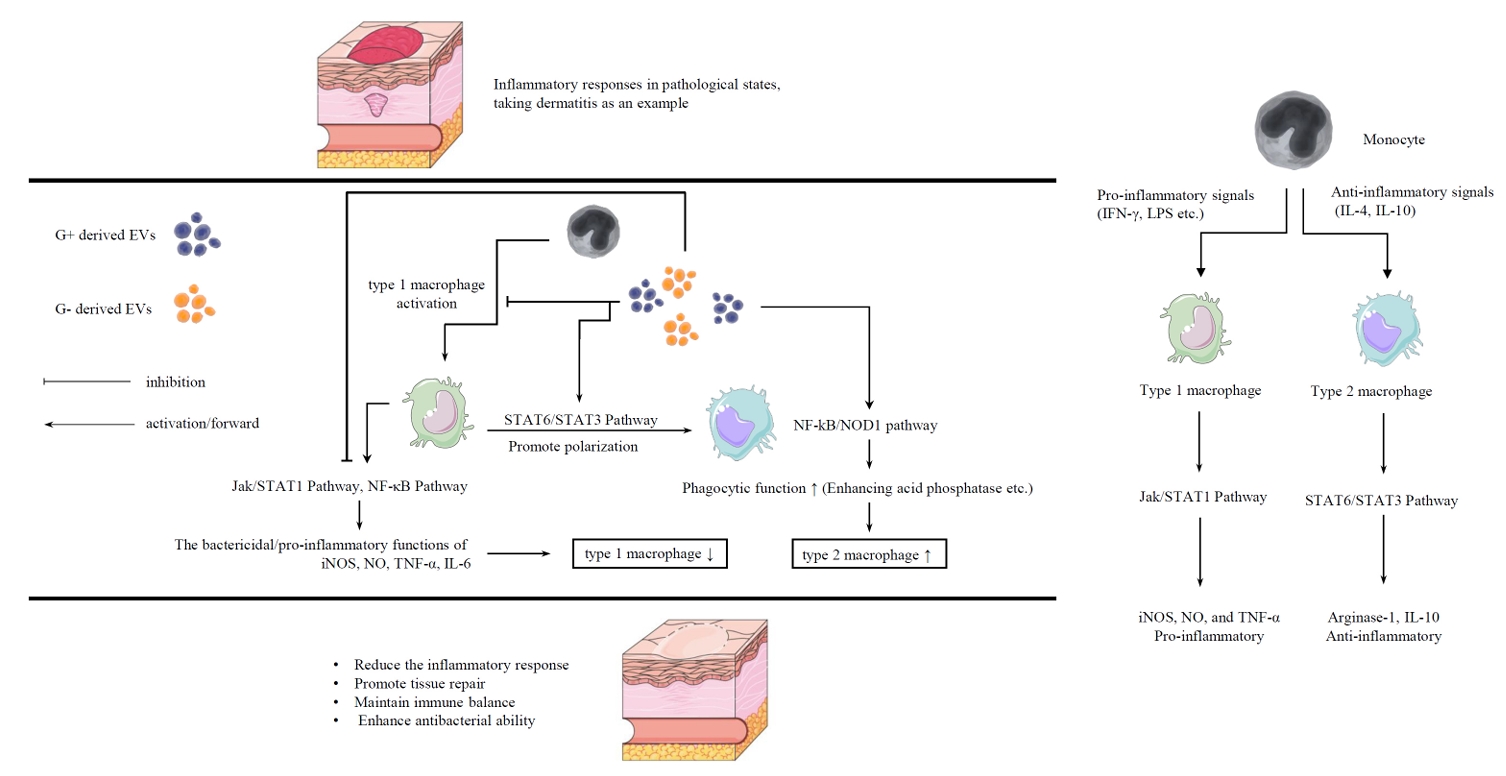
- Immunomodulatory Effects of Probiotic-derived EVs on Macrophages
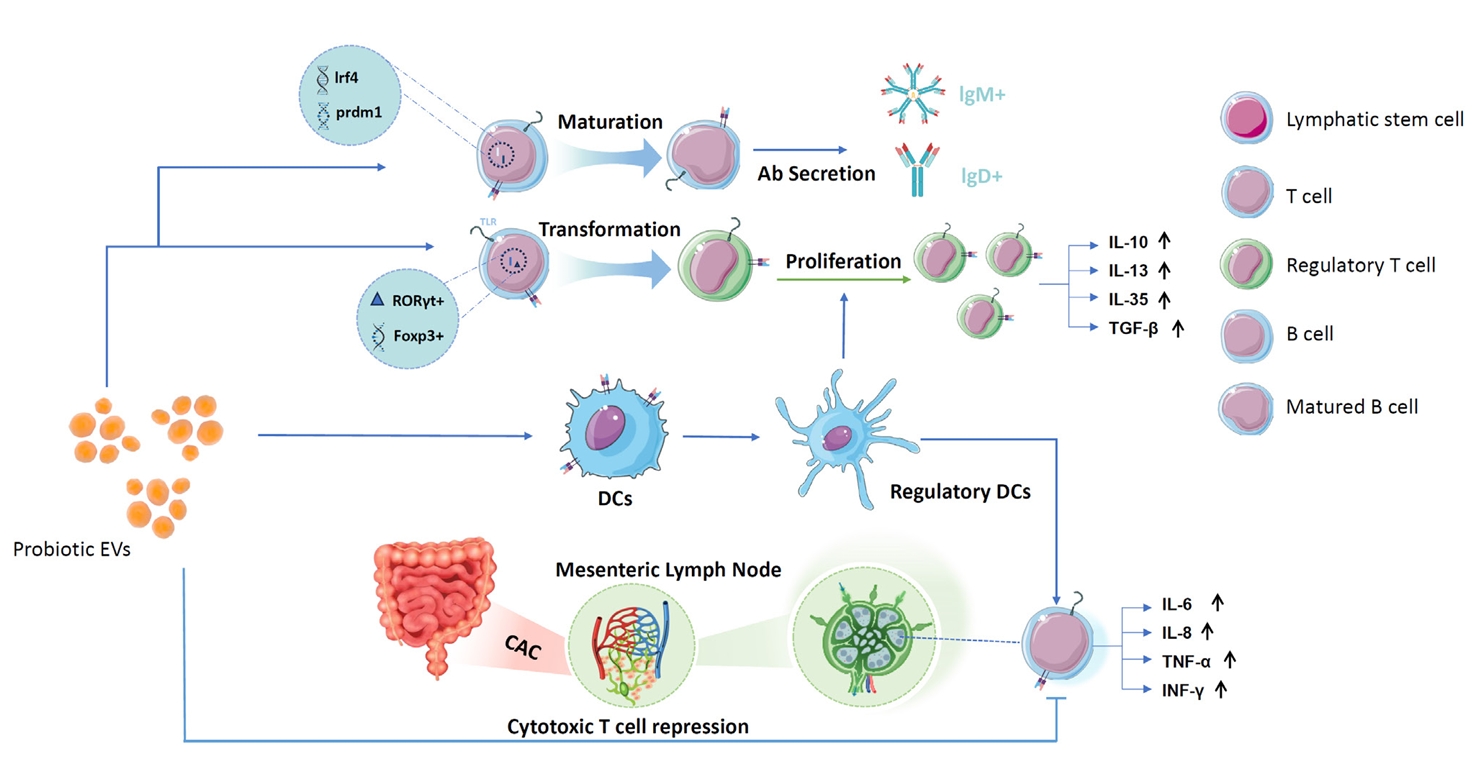
- Proliferation and Differentiation of Regulatory T Cells and Suppression of Cytotoxic T Cells
- Immune Intervention with B Cells
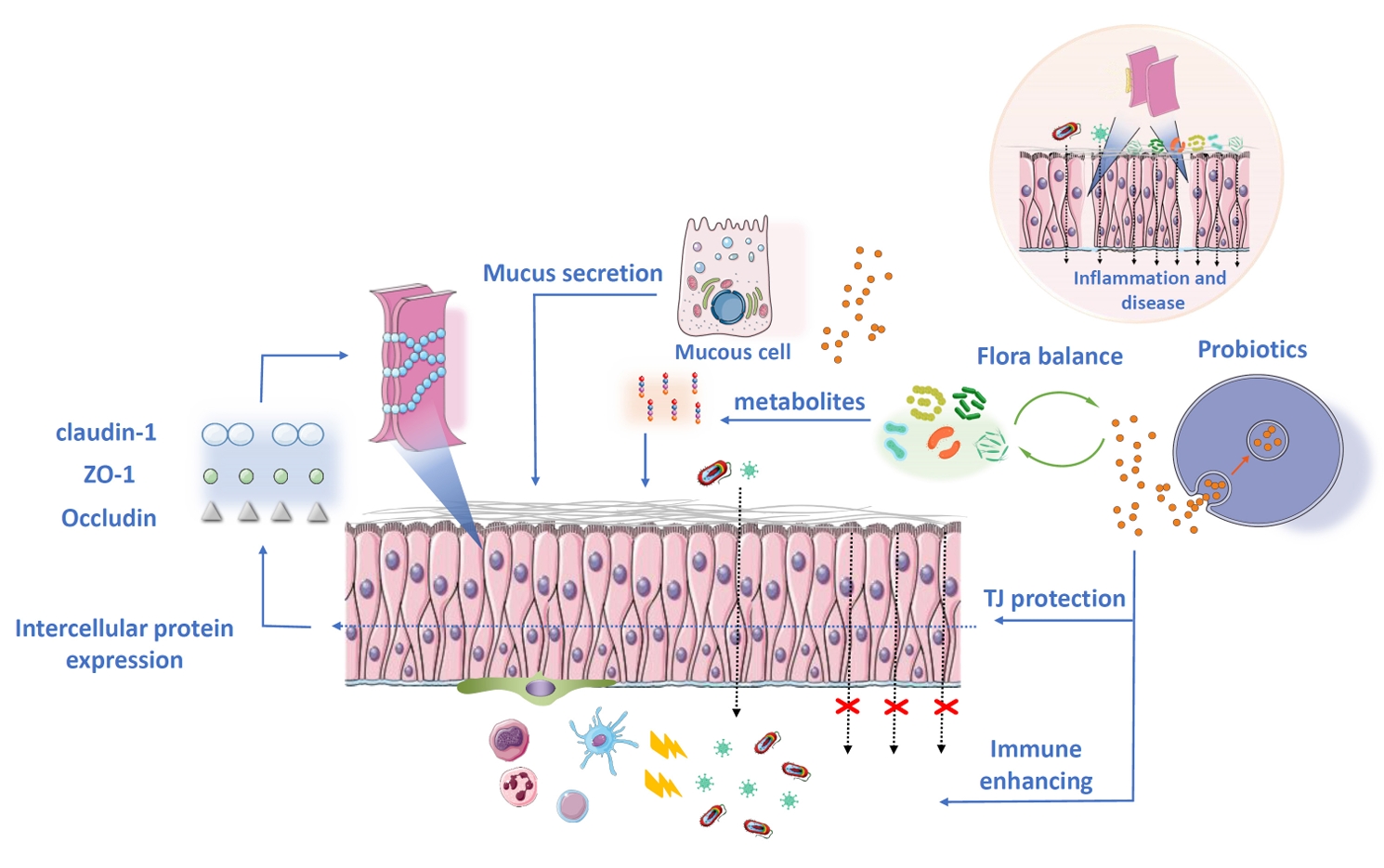
- Intervention of Probiotic EVs in Intestinal Barrier Protection
- Probiotic EVs Regulate Flora Interactions
- Antitumor Capability of Probiotic EVs
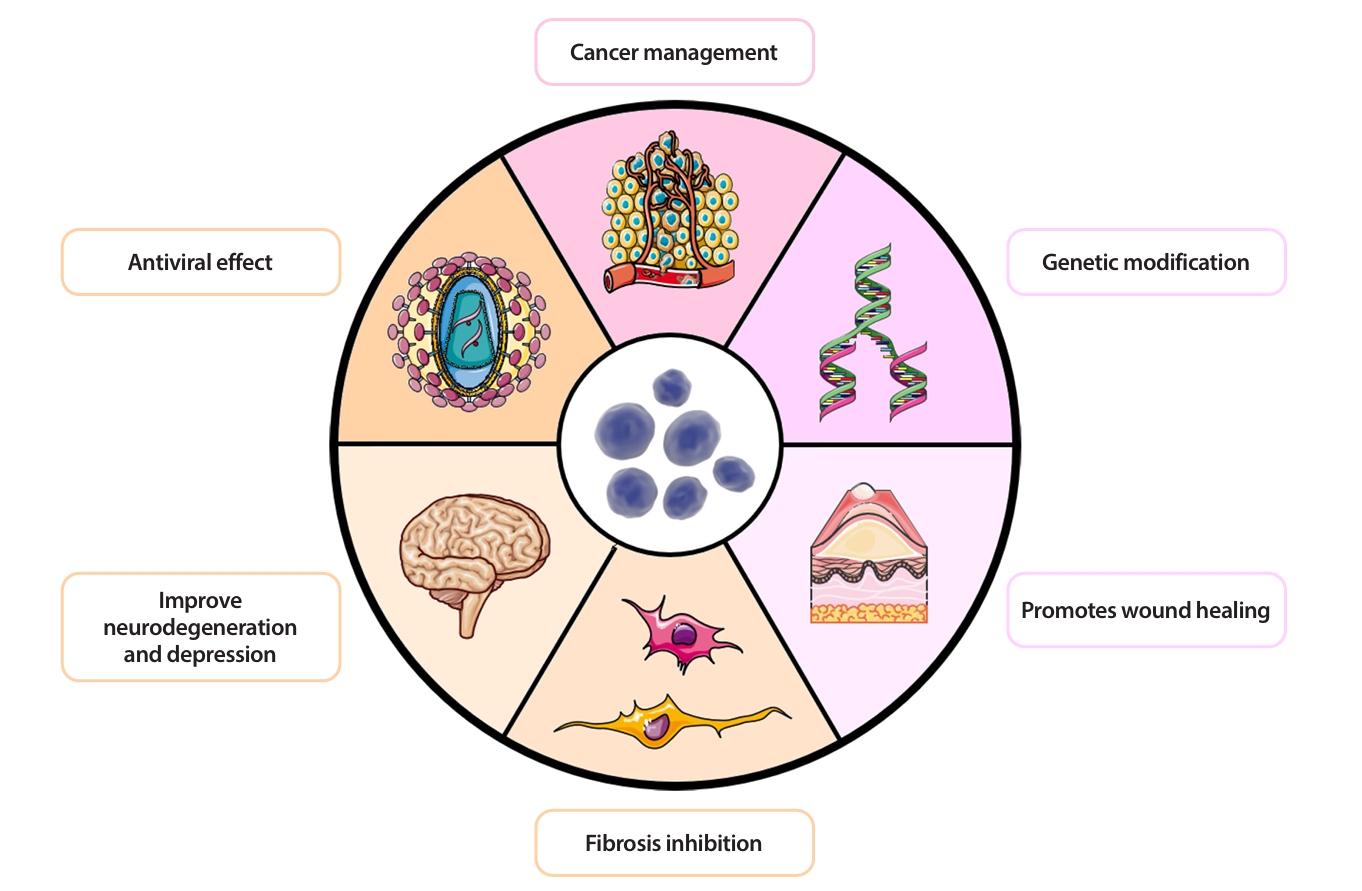
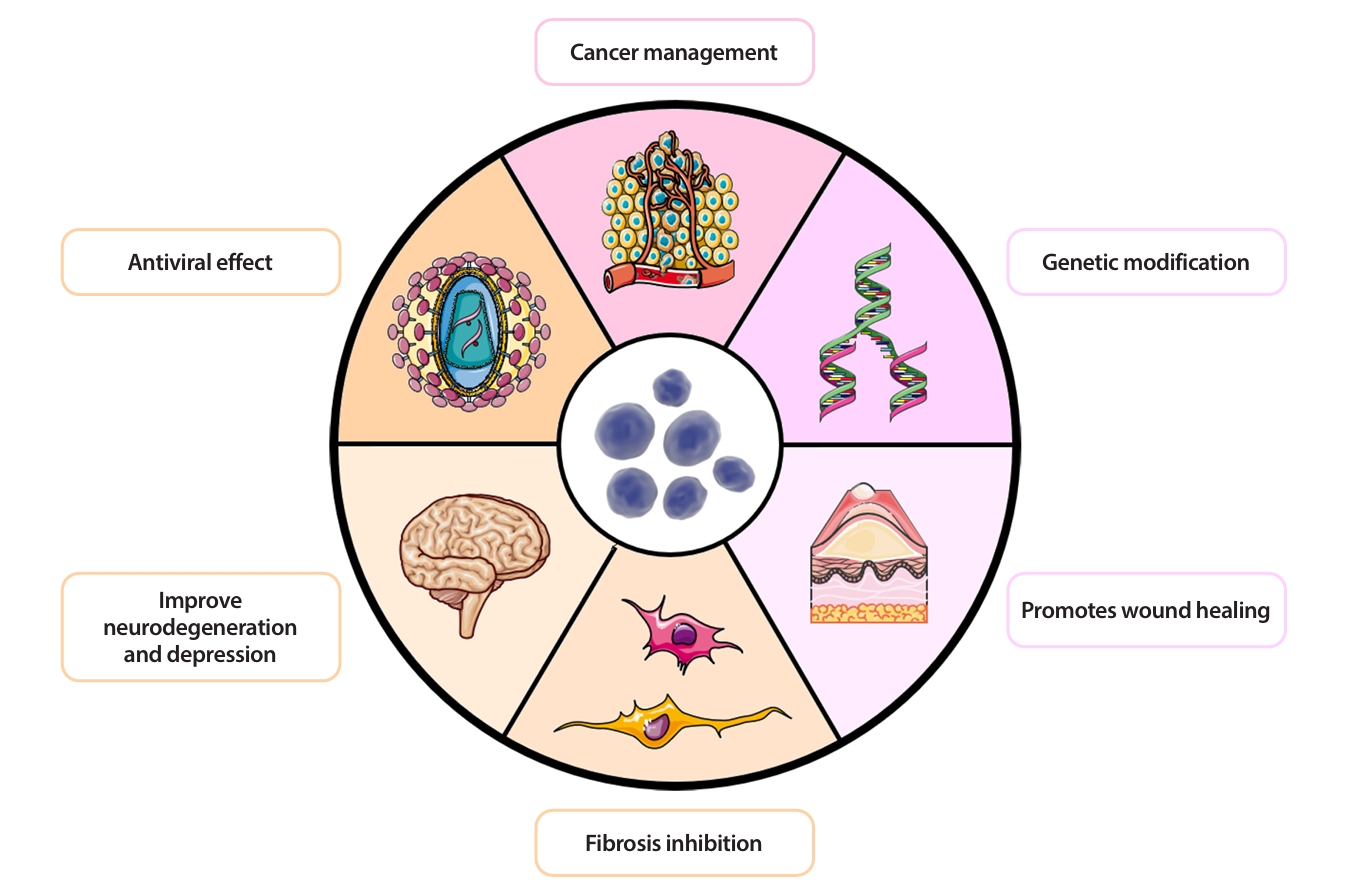
- More Biological Potential of Probiotic EVs
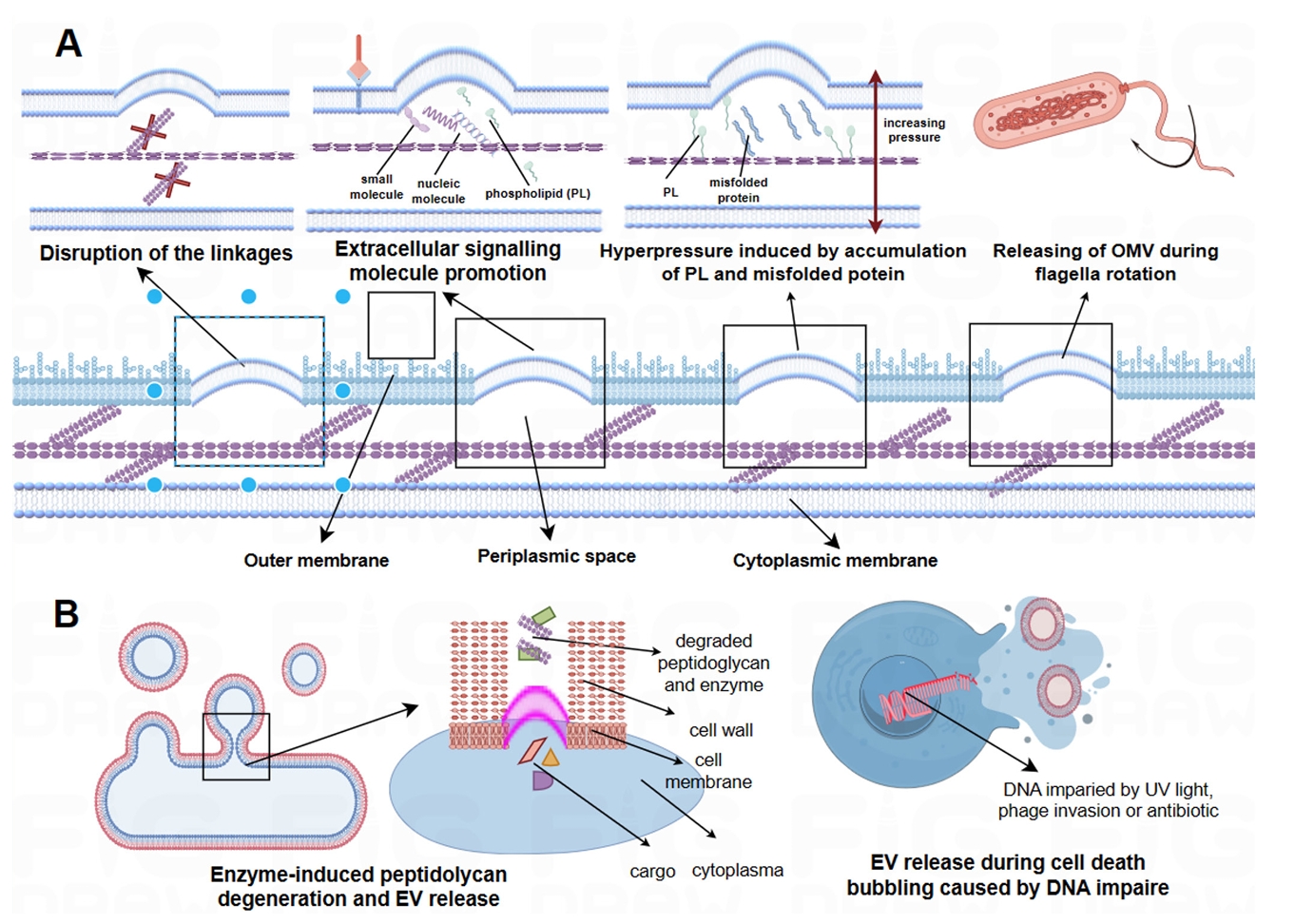
- EVs Biogenesis and Production
- The Stage of the Field, Limitations and Development
- Conclusion
- Notes
- References
ABSTRACT
- Extracellular vesicles derived from probiotics have received considerable attention for their pivotal role in bacterial‒host communication. These nanosized, bilayer-encapsulated vesicles carry diverse bioactive molecules, such as proteins, lipids, nucleic acids, and metabolites. Currently, ample evidence has emerged that probiotic extracellular vesicles may modulate several processes of host physiological hemostasis and offer therapeutic benefits. This review examines the biogenesis, composition, and immunomodulatory functions of probiotic-derived extracellular vesicles in probiotic–host interactions, highlighting the therapeutic potential of probiotic extracellular vesicles in the diagnosis and treatment of conditions such as cancer and inflammatory bowel disease. We further summarize the techniques for the separation and purification of extracellular vesicles, providing a methodological foundation for future research and applications. Although the field of probiotic extracellular vesicle research is still in its infancy, the prospects for their application in the biomedical field are broad, potentially emerging as a novel therapeutic approach.
Introduction
Biological Effects of Probiotic EVs
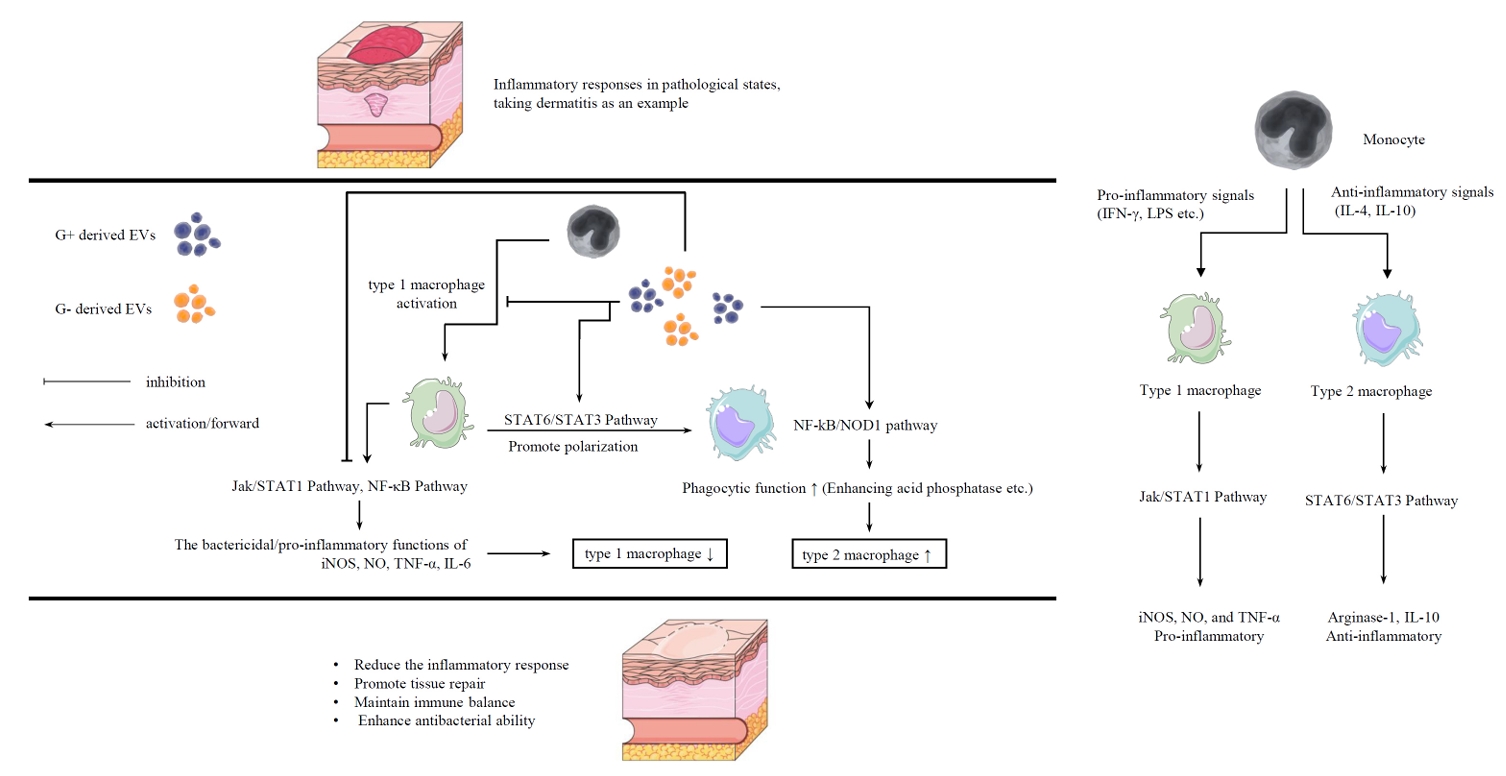
Immunomodulatory Effects of Probiotic-derived EVs on Macrophages
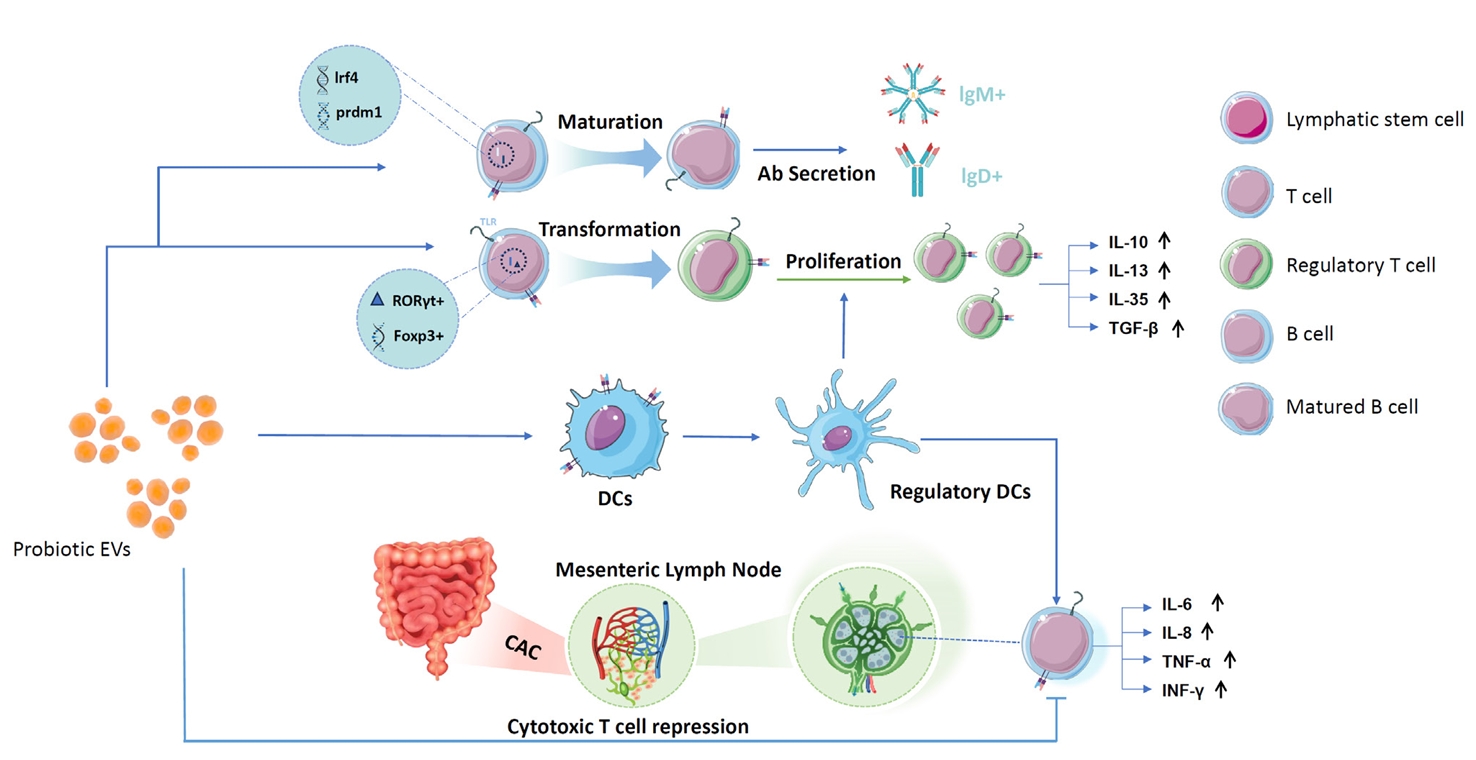
Proliferation and Differentiation of Regulatory T Cells and Suppression of Cytotoxic T Cells
Immune Intervention with B Cells
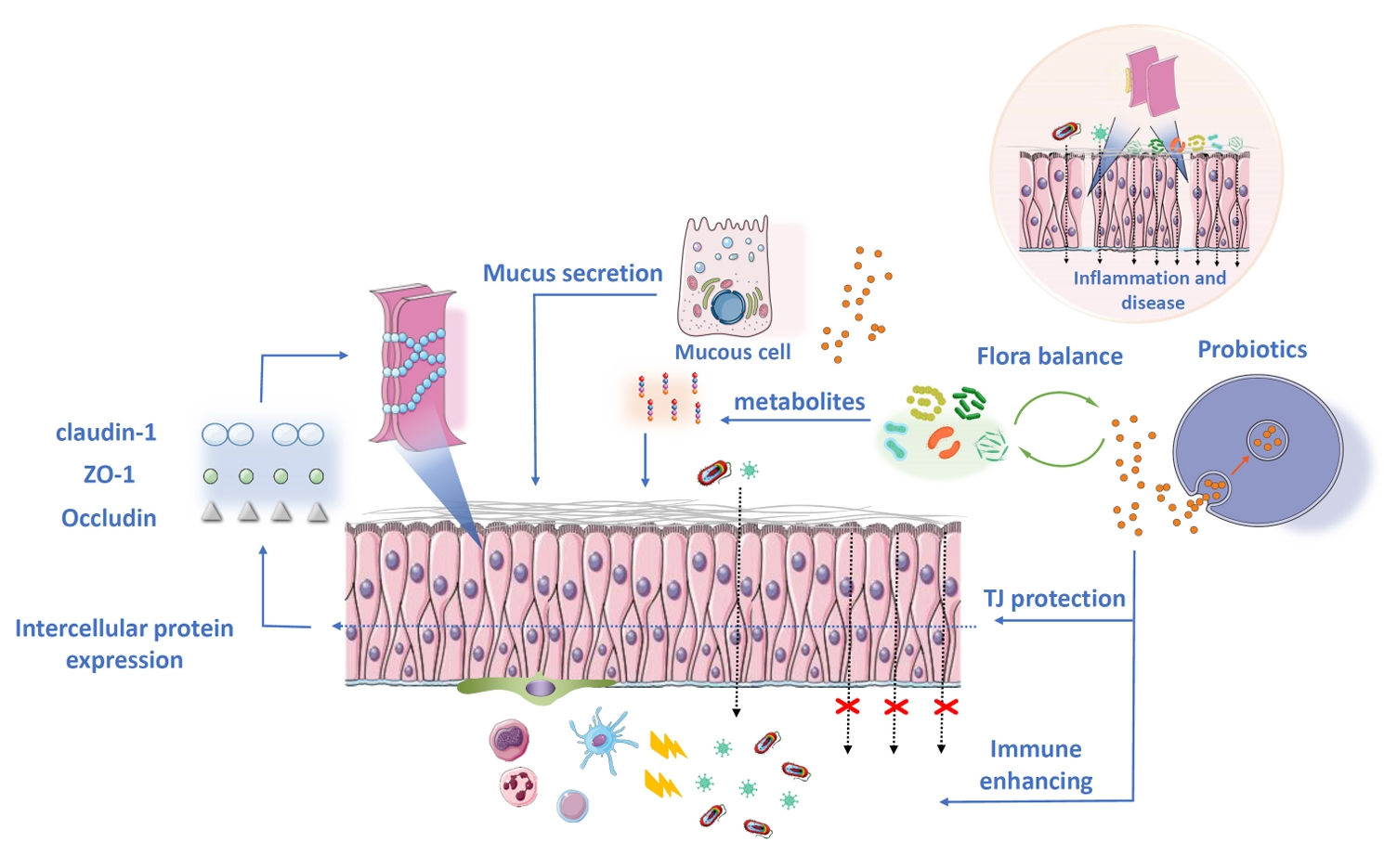
Intervention of Probiotic EVs in Intestinal Barrier Protection
Probiotic EVs Regulate Flora Interactions
Antitumor Capability of Probiotic EVs
More Biological Potential of Probiotic EVs
EVs Biogenesis and Production
The Stage of the Field, Limitations and Development
Conclusion
Acknowledgments
Financial acknowledgments: None.
Personal acknowledgments: This research would not have been possible without the support of many. We are grateful to the School of Basic Medicine at Nanchang University and Queen Mary College, Nanchang University. They provided a great academic environment, resources, facilities, and exchange opportunities, which helped our research.
Special thanks to the Pathogenic Microbiology Laboratory of the School of Basic Medicine, Nanchang University. Their platform support, with advanced equipment and good conditions, ensured that the research went smoothly. Additionally, we would like to thank everyone who contributed to this thesis, whether with academic ideas, technical help, or a good research environment. Their efforts made this research successful.
Author Contributions
YW, CD, and YM conceptualized the manuscript idea; YW and CD wrote the original draft and final manuscript; YW and CD prepared all the figures and tables; and YM Project administration. All the authors read and approved the final manuscript.
Conflict of Interest
None.
Funding
Not applicable.
Data Availability
Not applicable.
Ethical Statements
Not applicable.
- Ahmad MS, Krishnan S, Ramakrishna BS, Mathan M, Pulimood AB, et al. 2000. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut. 46: 493–499. ArticlePubMedPMC
- Batista JH, Leal FC, Fukuda TTH, Diniz JA, Almeida F, et al. 2020. Interplay between two quorum sensing-regulated pathways, violacein biosynthesis and VacJ/Yrb, dictates outer membrane vesicle biogenesis in Chromobacterium violaceum. Environ Microbiol. 22: 2432–2442. ArticlePubMed
- Battal B, Akgun V, Karaman B. 2014. Value of 3T 1H-magnetic resonance spectroscopy in the differentiation of benign and malignant breast tumors. Acta Radiol. 55: 416–417. ArticlePubMedLink
- Behrouzi A, Mazaheri H, Falsafi S, Tavassol ZH, Moshiri A, et al. 2020. Intestinal effect of the probiotic Escherichia coli strain Nissle 1917 and its OMV. J Diabetes Metab Disord. 19: 597–604. ArticlePubMedPMCPDF
- Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, et al. 2021. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 39: 708–724. ArticlePubMed
- Brown L, Kessler A, Cabezas-Sanchez P, Luque-Garcia JL, Casadevall A. 2014. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol Microbiol. 93: 183–198. ArticlePubMedPMC
- Browne D. 2023. Cancer is the second leading cause of death in the U.S. men and women. J Natl Med Assoc. 115: S1.ArticlePubMed
- Cañas MA, Giménez R, Fábrega MJ, Toloza L, Baldomà L. 2016. Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS One. 11: e0160374. ArticlePubMedPMC
- Cao X. 2016. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 16: 35–50. ArticlePubMedPDF
- Choi J, Kim YK, Han PL. 2019. Extracellular vesicles derived from Lactobacillus plantarum increase BDNF expression in cultured hippocampal neurons and produce antidepressant-like effects in mice. Exp Neurobiol. 28: 158–171. ArticlePubMedPMCLink
- Choi J, Kwon H, Kim YK, Han PL. 2022. Extracellular vesicles from Gram-positive and Gram-negative probiotics remediate stress-induced depressive behavior in mice. Mol Neurobiol. 59: 2715–2728. ArticlePubMed
- Deng XY, Li HY, Chen JJ, Li RP, Qu R, et al. 2015. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav Brain Res. 291: 12–19. ArticlePubMed
- Ding Y, Bu F, Chen T, Shi G, Yuan X, et al. 2021. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. 1051: 8411–8426. ArticlePDF
- Ermann Lundberg L, Pallabi Mishra P, Liu P, Forsberg MM, Sverremark-Ekström E, et al. 2024. Bifidobacterium longum subsp. longum BG-L47 boosts growth and activity of Limosilactobacillus reuteri DSM 17938 and its extracellular membrane vesicles. Appl Environ Microbiol. 90: e0024724. ArticlePubMed
- Fábrega MJ, Aguilera L, Giménez R, Varela E, Cañas MA, et al. 2016. Activation of immune and defense responses in the intestinal mucosa by outer membrane vesicles of commensal and probiotic Escherichia coli strains. Front Microbiol. 7: 705.ArticlePubMedPMC
- Fábrega MJ, Rodríguez-Nogales A, Garrido-Mesa J, Algieri F, Badía J, et al. 2017. Intestinal anti-inflammatory effects of outer membrane vesicles from Escherichia coli Nissle 1917 in DSS-experimental colitis in mice. Front Microbiol. 8: 1274.ArticlePubMedPMC
- Fan J, Zhang Y, Zuo M, Ding S, Li J, et al. 2024. Novel mechanism by which extracellular vesicles derived from Lactobacillus murinus alleviates deoxynivalenol-induced intestinal barrier disruption. Environ Int. 185: 108525.ArticlePubMed
- Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. 2017. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 3: 524–548. ArticlePubMedPMC
- Furuyama N, Sircili MP. 2021. Outer membrane vesicles (OMVs) produced by Gram-negative bacteria: Structure, functions, biogenesis, and vaccine application. Biomed Res Int. 2021: 1490732.ArticlePubMedPMCPDF
- Gerondakis S, Fulford TS, Messina NL, Grumont RJ. 2014. NF-κB control of T cell development. Nat Immunol. 15: 15–25. ArticlePubMedPDF
- Gong L, Zhang Y, Liu C, Zhang M, Han S. 2021. Application of radiosensitizers in cancer radiotherapy. Int J Nanomedicine. 16: 1083–1102. ArticlePubMedPMCLink
- Guo Q, Jin Y, Chen X, Ye X, Shen X, et al. 2024. NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct Target Ther. 9: 53.ArticlePubMedPMCPDF
- Han F, Wang K, Shen K, Wang J, Han S, et al. 2023. Extracellular vesicles from Lactobacillus druckerii inhibit hypertrophic scar fibrosis. J Nanobiotechnol. 21: 113.ArticlePDF
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: The next generation. Cell. 144: 646–674. ArticlePubMed
- Hass R, Busche R, Luciano L, Reale E, Engelhardt WV. 1997. Lack of butyrate is associated with induction of Bax and subsequent apoptosis in the proximal colon of guinea pig. Gastroenterology. 112: 875–881. ArticlePubMed
- Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, et al. 2021. Bifidobacteria-mediated immune system imprinting early in life. Cell. 1845: 3884–3898. Article
- Hiippala K, Barreto G, Burrello C, Diaz-Basabe A, Suutarinen M, et al. 2020. Novel Odoribacter splanchnicus strain and its outer membrane vesicles exert immunoregulatory effects in vitro. Front Microbiol. 11: 575455.ArticlePubMedPMC
- Housmans BAC, Neefjes M, Surtel DAM, Vitík M, Cremers A, et al. 2022. Synovial fluid from end-stage osteoarthritis induces proliferation and fibrosis of articular chondrocytes via MAPK and RhoGTPase signaling. Osteoarthritis Cartilage. 30: 862–874. ArticlePubMed
- Hu R, Lin H, Li J, Zhao Y, Wang M, et al. 2020. Probiotic Escherichia coli Nissle 1917-derived outer membrane vesicles enhance immunomodulation and antimicrobial activity in RAW264.7 macrophages. BMC Microbiol. 20: 268.ArticlePubMedPMCPDF
- Jach ME, Serefko A, Szopa A, Sajnaga E, Golczyk H, et al. 2023. The role of probiotics and their metabolites in the treatment of depression. Molecules. 28: 3213.ArticlePubMedPMC
- Jiang B, Huang J. 2024. Influences of bacterial extracellular vesicles on macrophage immune functions. Front Cell Infect Microbiol. 14: 1411196.ArticlePubMedPMC
- Jin Y, Ma L, Zhang W, Yang W, Feng Q, et al. 2022. Extracellular signals regulate the biogenesis of extracellular vesicles. Biol Res. 55: 35.ArticlePubMedPMCPDF
- Karamitopoulou E, Wenning AS, Acharjee A, Zlobec I, Aeschbacher P. 2023. Spatially restricted tumour-associated and host-associated immune drivers correlate with the recurrence sites of pancreatic cancer. Gut. 72: 1523–1533. ArticlePubMed
- Katsir L, Bahar O. 2017. Bacterial outer membrane vesicles at the plant-pathogen interface. PLoS Pathog. 13: e1006306. ArticlePubMedPMC
- Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Hupp T, Duchnowska R, Marek-Trzonkowska N, et al. 2022. Next-generation probiotics - do they open new therapeutic strategies for cancer patients? Gut Microbes. 14: 2035659.ArticlePubMedPMC
- Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, et al. 2017. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 8: 626.ArticlePubMedPMCPDF
- Kumar P, Zadjali F, Yao Y, Köttgen M, Hofherr A, et al. 2022. Single gene mutations in Pkd1 or Tsc2 alter extracellular vesicle production and trafficking. Biology. 11: 709.ArticlePubMedPMC
- Kurata A, Kiyohara S, Imai T, Yamasaki-Yashiki S, Zaima N, et al. 2022. Characterization of extracellular vesicles from Lactiplantibacillus plantarum. Sci Rep. 12: 13330.ArticlePubMedPMCPDF
- Kwon H, Lee EH, Park SY, Park JY, Hong JH, et al. 2023. Lactobacillus-derived extracellular vesicles counteract Aβ42-induced abnormal transcriptional changes through the upregulation of MeCP2 and Sirt1 and improve Aβ pathology in Tg-APP/PS1 mice. Exp Mol Med. 55: 2067–2082. ArticlePubMedPMCPDF
- Lee XR, Xiang GL. 2018. Effects of edaravone, the free radical scavenger, on outcomes in acute cerebral infarction patients treated with ultra-early thrombolysis of recombinant tissue plasminogen activator. Clin Neurol Neurosurg. 167: 157–161. ArticlePubMed
- Li Y, Zhao R, Cheng K, Zhang K, Wang Y, et al. 2020. Bacterial outer membrane vesicles presenting programmed death 1 for improved cancer immunotherapy via immune activation and checkpoint inhibition. ACS Nano. 14: 16698–16711. ArticlePubMed
- Liang S, Wu X, Hu X, Wang T, Jin F. 2018. Recognizing depression from the microbiota-gut-brain axis. Int J Mol Sci. 19: 1592.ArticlePubMedPMC
- Liu Y, Chen J, Raj K, Baerg L, Nathan N, et al. 2023. A universal strategy to promote secretion of G+/G- bacterial extracellular vesicles and its application in host innate immune responses. ACS Synth Biol. 12: 319–328. ArticlePubMedLink
- Liu L, Guo H, Song A, Huang J, Zhang Y, et al. 2020. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-κB and MAPK pathways. BMC Immunol. 21: 32.ArticlePubMedPMCPDF
- Liu YC, Wu CR, Huang TW. 2022. Preventive effect of probiotics on oral mucositis induced by cancer treatment: a systematic review and meta-analysis. Int J Mol Sci. 23: 13268.ArticlePubMedPMC
- Lu S, Xu J, Zhao Z, Guo Y, Zhang H, et al. 2023. Dietary Lactobacillus rhamnosus GG extracellular vesicles enhance antiprogrammed cell death 1 (anti-PD-1) immunotherapy efficacy against colorectal cancer. Food Funct. 143: 10314–10328. Article
- Ma J, Sun L, Liu Y, Ren H, Shen Y, et al. 2020. Alter between gut bacteria and blood metabolites and the anti-tumor effects of Faecalibacterium prausnitzii in breast cancer. BMC Microbiol. 20: 82.ArticlePubMedPMCPDF
- Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, et al. 2020. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 369: 1481–1489. ArticlePubMed
- Manning AJ, Kuehn MJ. 2013. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J Mol Microbiol Biotechnol. 23: 131–141. ArticlePubMedLink
- Maomao C, He L, Dianqin S, Siyi H, Xinxin Y, et al. 2022. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. 19: 1121–1138. ArticlePubMedPMC
- Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. 2021. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 80: 37–49. ArticlePubMed
- Mitsuma A, Ando Y. 2022. Chemotherapy for older patients with cancer. Gan To Kagaku Ryoho. 49: 13–18. PubMed
- Mosby CA, Bhar S, Phillips MB, Edelmann MJ, Jones MK. 2022. Interaction with mammalian enteric viruses alters outer membrane vesicle production and content by commensal bacteria. J Extracell Vesicles. 11: e12172. ArticlePubMedPMCLink
- Ñahui Palomino RA, Vanpouille C, Laghi L, Parolin C, Melikov K, et al. 2019. Extracellular vesicles from symbiotic vaginal lactobacilli inhibit HIV-1 infection of human tissues. Nat Commun. 10: 5656.PubMedPMC
- Nozaki K, Li L, Miao EA. 2022. Innate sensors trigger regulated cell death to combat intracellular infection. Annu Rev Immunol. 40: 469–498. ArticlePubMedPMC
- Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, et al. 2017. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE. 12: e0173004. Article
- Perdiguero P, Martín-Martín A, Benedicenti O, Díaz-Rosales P, Morel E, et al. 2019. Teleost IgD⁺IgM⁻ B cells mount clonally expanded and mildly mutated intestinal IgD responses in the absence of lymphoid follicles. Cell Rep. 293: 4223–4235. Article
- Peregrino ES, Castaneda-Casimiro J, Vazquez-Flores L, Estrada-Parra S, Wong-Baeza C, et al. 2024. The role of bacterial extracellular vesicles in the immune response to pathogens, and therapeutic opportunities. Int J Mol Sci. 25: 6210.ArticlePubMedPMC
- Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. 2014. NOD proteins: Regulators of inflammation in health and disease. Nat Rev Immunol. 14: 9–23. ArticlePubMedPDF
- Qian L, Qian C, Chen Y, Bai Y, Bao Y, et al. 2012. Regulatory dendritic cells program B cells to differentiate into CD19hiFcγIIbhi regulatory B cells through IFN-β and CD40L. Blood. 120: 581–591. ArticlePubMedPDF
- Qiu-Sha P, Shi-bing S, Ming Z. 2019. Advances in functional studies of probiotic Escherichia coli Nissle1917. Microbiol China. 46: 3133–3139.
- Resch U, Tsatsaronis JA, Le Rhun A, Stübiger G, Rohde M, et al. 2016. A two-component regulatory system impacts extracellular membrane-derived vesicle production in group A Streptococcus. mBio. 7: e00207–16. ArticlePubMedPMCLink
- Round JL, Mazmanian SK. 2010. Inducible Foxp3⁺ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 107: 12204–12209. ArticlePubMedPMC
- Rožman P, Švajger U. 2018. The tolerogenic role of IFN-γ. Cytokine Growth Factor Rev. 41: 40–53. ArticlePubMed
- Sandanusova M, Turkova K, Pechackova E, Kotoucek J, Roudnicky P, et al. 2024. Growth phase matters: Boosting immunity via Lacticasebacillus-derived membrane vesicles and their interactions with TLR2 pathways. J Extracell Biol. 3: e169. ArticlePubMedPMC
- Sangiorgio G, Nicitra E, Bivona D, Bonomo C, Bonacci P, et al. 2024. Interactions of Gram-positive bacterial membrane vesicles and hosts: updates and future directions. Int J Mol Sci. 25: 2904.ArticlePubMedPMC
- Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, et al. 2012. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 12: 509–520. ArticlePubMedPMC
- Spencer RL, Deak T. 2017. A users guide to HPA axis research. Physiol Behav. 178: 43–65. ArticlePubMed
- Stentz R, Horn N, Cross K, Salt L, Brearley C, et al. 2014. Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp. protect gut pathogens and commensals against β-lactam antibiotics. J Antimicrob Chemother. 70: 701–709. ArticlePubMedPMC
- Strzelec M, Detka J, Mieszczak P, Sobocińska MK, Majka M. 2023. Immunomodulation—a general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front Immunol. 14: 1127704.ArticlePubMedPMC
- Sun E, Meng X, Kang Z, Gu H, Li M, et al. 2023. Zengshengping improves lung cancer by regulating the intestinal barrier and intestinal microbiota. Front Pharmacol. 14: 1123819.ArticlePubMedPMC
- Takiguchi N, Soda H, Tonooka T, Nabeya Y, Hoshino I, et al. 2020. Significance of surgery for multidisciplinary treatment including neoadjuvant chemotherapy for locally advanced colorectal cancer. Gan To Kagaku Ryoho. 473: 2174–2176.
- Thomas CM, Versalovic J. 2010. Probiotics-host communication: Modulation of signaling pathways in the intestine. Gut Microbes. 1: 148–163. ArticlePubMedPMC
- Thomsen M, Vitetta L. 2018. Adjunctive treatments for the prevention of chemotherapy- and radiotherapy-induced mucositis. Integr Cancer Ther. 17: 1027–1047. ArticlePubMedPMCLink
- Tomasi M, Caproni E, Benedet M, Zanella I, Giorgetta S, et al. 2022. Outer membrane vesicles from the gut microbiome contribute to tumor immunity by eliciting cross-reactive T cells. Front Oncol. 12: 912639.ArticlePubMedPMC
- Ton-That H, Marraffini LA, Schneewind O. 2004. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim Biophys Acta. 1694: 269–278. ArticlePubMed
- Toyofuku M, Nomura N, Eberl L. 2019. Types and origins of bacterial membrane vesicles. Nat Rev Microbiol. 17: 13–24. ArticlePubMedPDF
- van Zyl WF, Deane SM, Dicks LMT. 2020. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes. 12: 1831339.ArticlePubMedPMC
- Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. 2017. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 198: 1006–1014. ArticlePubMedPDF
- Vicente-Gil S, Nuñez-Ortiz N, Morel E, Serra CR, Docando F, et al. 2024. Immunomodulatory properties of Bacillus subtilis extracellular vesicles on rainbow trout intestinal cells and splenic leukocytes. Front Immunol. 15: 1394501.ArticlePubMedPMC
- Wang S, Guo J, Bai Y, Sun C, Wu Y, et al. 2022. Bacterial outer membrane vesicles as a candidate tumor vaccine platform. Front Immunol. 13: 987419.ArticlePubMedPMC
- Wang N, Liang H, Zen K. 2014. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol. 5: 614.ArticlePubMedPMC
- Weyant KB, Oloyede A, Pal S, Liao J, Jesus MRD, et al. 2023. A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens. Nat Commun. 14: 464.ArticlePubMedPMCPDF
- Xia C, Dong X, Li H, Cao M, Sun D, et al. 2022. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 135: 584–590. ArticlePubMedPMC
- Xu X, Lv J, Guo F, Li J, Jia Y, et al. 2020. Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front Microbiol. 11: 814.ArticlePubMedPMC
- Xue M, Ji X, Liang H, Liu Y, Wang B, et al. 2018. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 9: 1214–1223. ArticlePubMed
- Yu S, Zhang X, Li W, Lu Y, Xu X, et al. 2024. Thermosensitive hydrogel as a sustained release carrier for mesenchymal stem cell-derived extracellular vesicles in the treatment of intrauterine adhesion. J Nanobiotechnology. 22: 570.ArticlePubMedPMCPDF
- Yuan X, Chen B, Duan Z, Xia Z, Ding Y, et al. 2021. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes. 13: 1987779.ArticlePubMedPMC
- Yunna C, Mengru H, Lei W, Weidong C. 2020. Macrophage M1/M2 polarization. Eur J Pharmacol. 877: 173090.ArticlePubMed
- Zahmatkesh ME, Jahanbakhsh M, Hoseini N, Shegefti S, Peymani A, et al. 2022. Effects of exosomes derived from Helicobacter pylori outer membrane vesicle-infected hepatocytes on hepatic stellate cell activation and liver fibrosis induction. Front Cell Infect Microbiol. 12: 857570.ArticlePubMedPMC
- Zhou L, Foster JA. 2015. Psychobiotics and the gut-brain axis: In the pursuit of happiness. Neuropsychiatr Dis Treat. 11: 715–723. ArticlePubMedPMC
- Zhou X, Li X, Wu M. 2018. miRNAs reshape immunity and inflammatory responses in bacterial infection. Signal Transduct Target Ther. 3: 14.ArticlePubMedPMCPDF
- Zhu R, Zhang Y, Wang X, Liu BD, Chowdhury D, et al. 2024. Probiotic bacteria-released extracellular vesicles enhance macrophage phagocytosis in polymicrobial sepsis by activating the FPR1/2 pathway. Mol Med. 30: 216.ArticlePubMedPMCPDF
- Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, et al. 2007. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 9: 804–816. ArticlePubMed
References
Figure & Data
References
Citations

- Decoding bacterial extracellular vesicles: A review on isolation and characterization techniques
Malatesh S. Devati, Apoorva Jnana, Stephen P. Kidd, Slade O. Jensen, T. G. Satheesh Babu, Dinesh Upadhya, Thokur S. Murali
Archives of Microbiology.2026;[Epub] CrossRef - The supernatant of Lactiplantibacillus plantarum 25 is more effective than extracellular vesicles in alleviating ulcerative colitis and improving intestinal barrier function
Shuang Gong, Xin Li, Qiong Zhang, Rui Wang, Ruixia Zeng, Yibo Zhang
Frontiers in Microbiology.2026;[Epub] CrossRef - Standardizing Bacterial Extracellular Vesicle Purification: A Call for Consensus
Dongsic Choi, Eun-Young Lee
Journal of Microbiology and Biotechnology.2025;[Epub] CrossRef - Advances in Biological Functions and Applications of Feeding Microorganism-derived Extracellular Vesicles
Yuanyuan Zhu, Xiaofang Zhang, Xin Feng, Yanyan Huang, Langhong Wang, Huihua Zhang, Xinan Zeng, Zhonglin Tang, Qien Qi
Probiotics and Antimicrobial Proteins.2025;[Epub] CrossRef
 ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
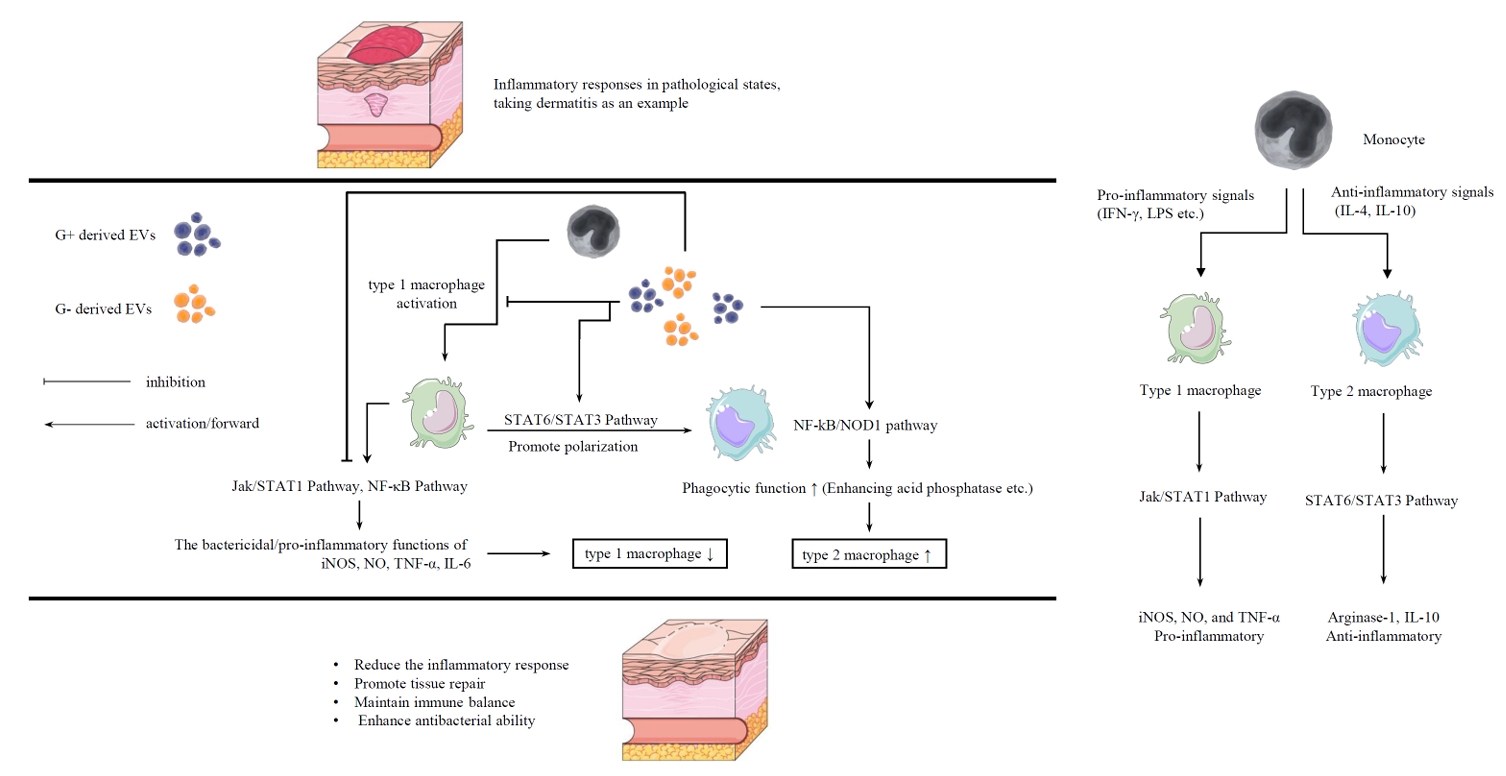
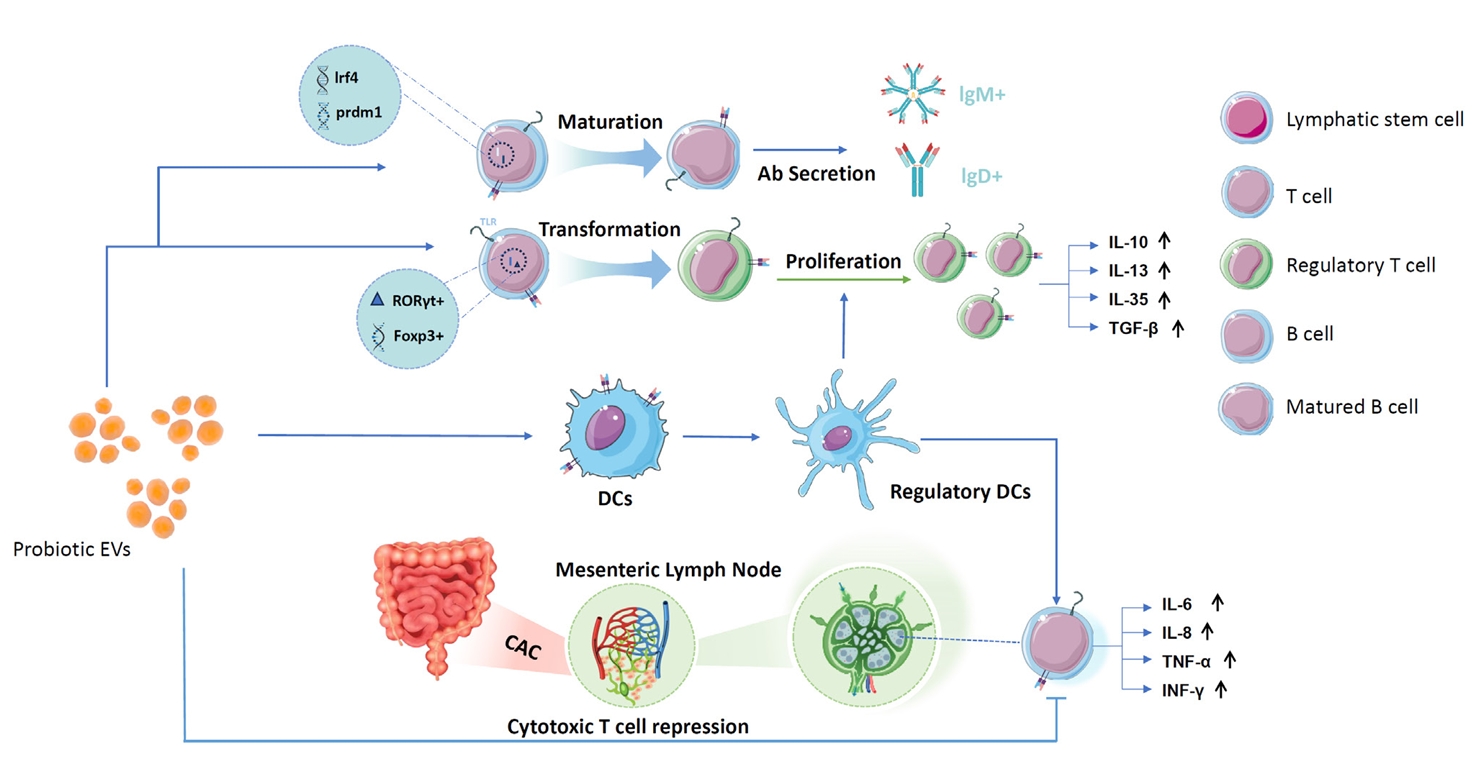
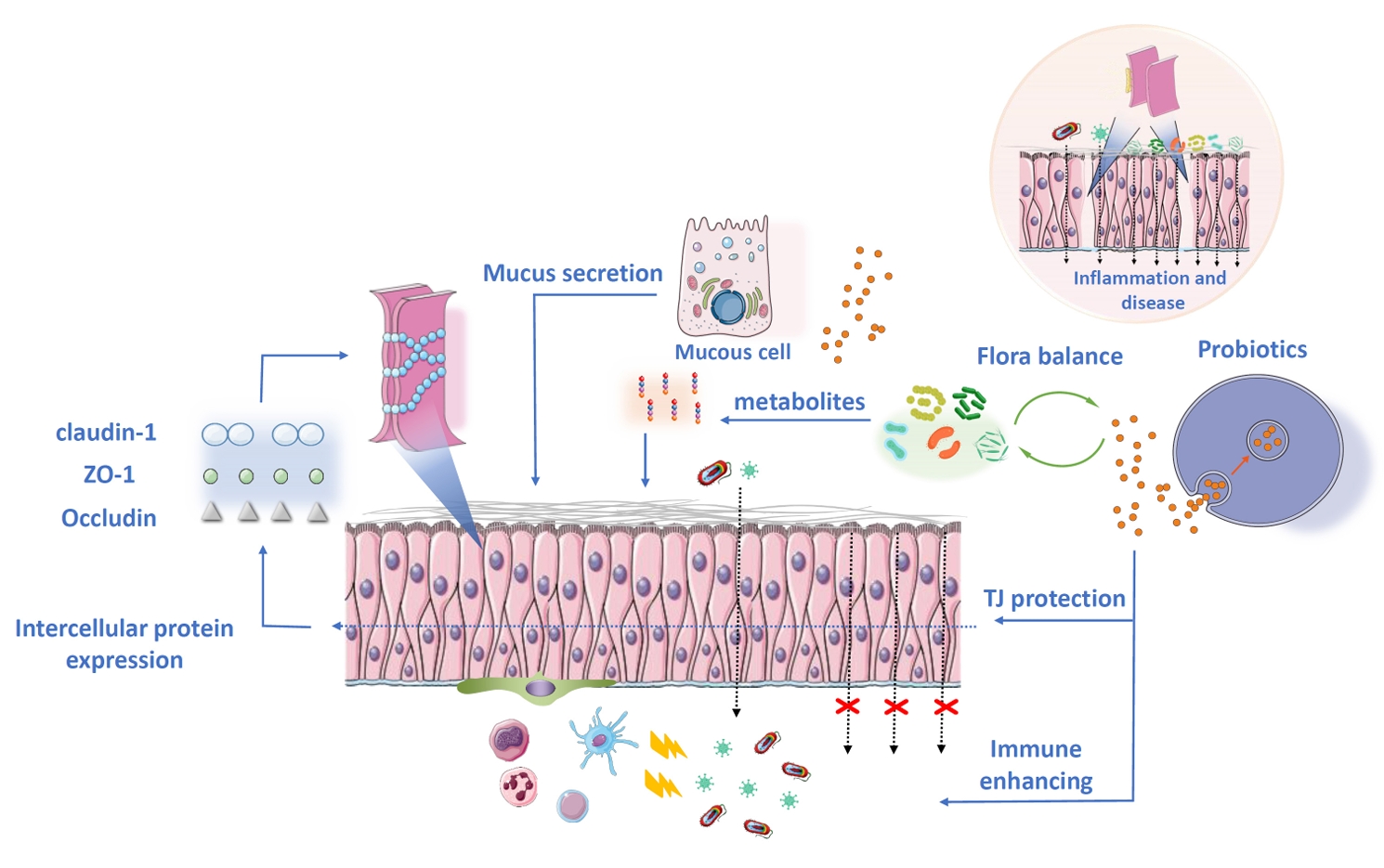
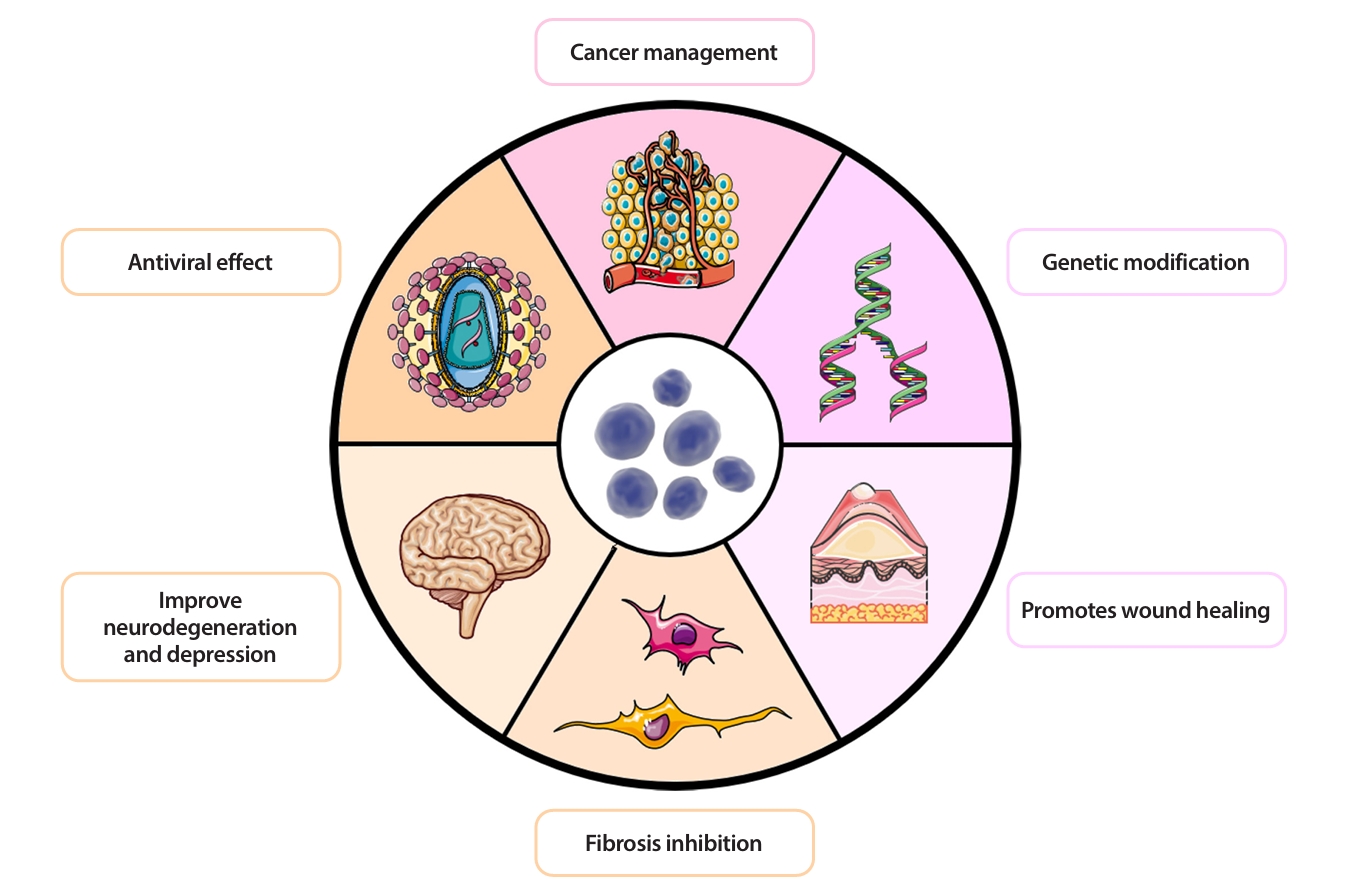
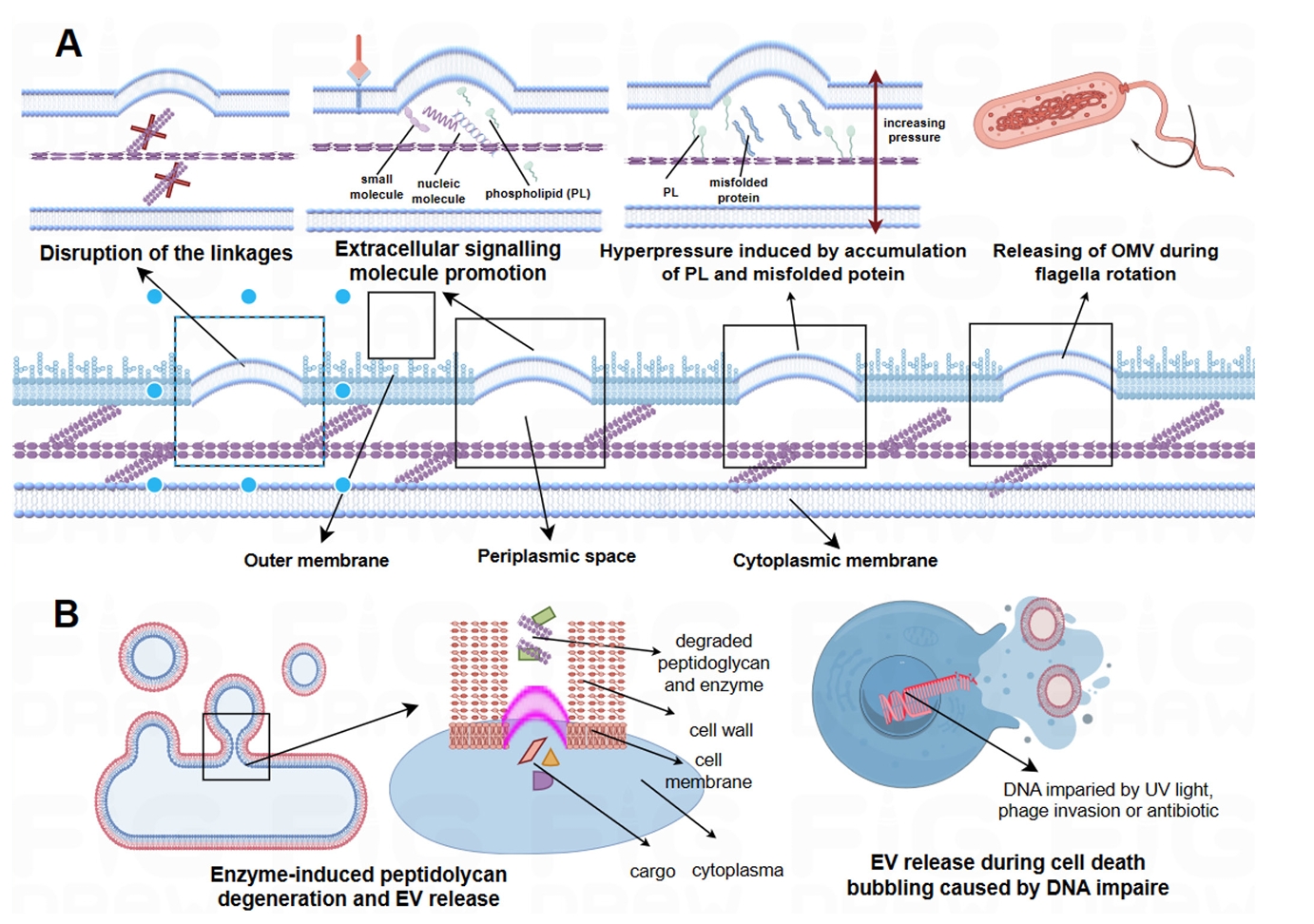
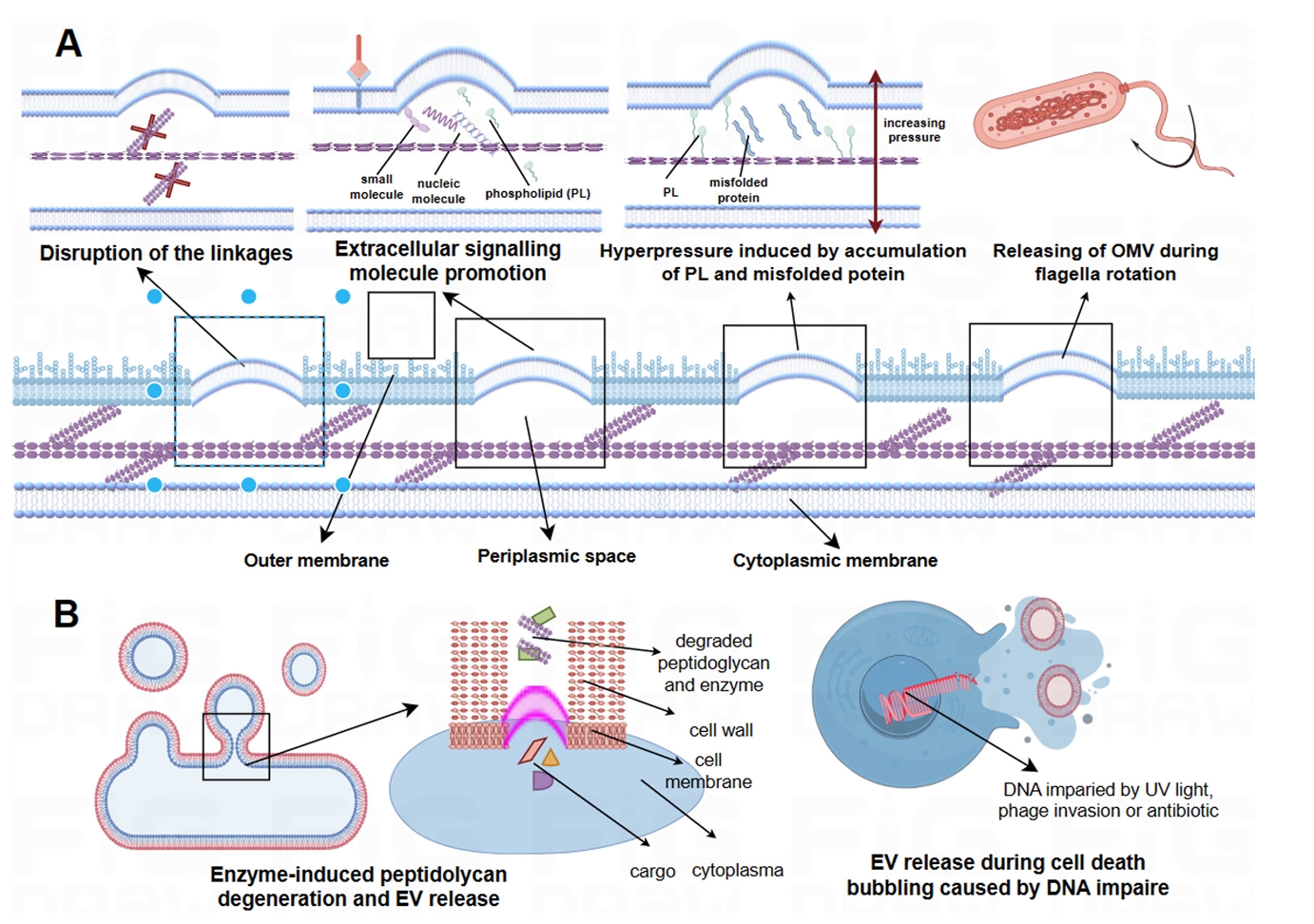
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
| Bacterial species | Classification | Potential application |
|---|---|---|
| A. muciniphila | G- | Metabolism improvement in obese |
| Intestinal barrier enhancement | ||
| Anti-inflammation | ||
| Immunoregulatory function | ||
| Dysbiosis control | ||
| Anti-tumor capability | ||
| Developing drug delivery system | ||
| O. splanchnicus | G- | Anti-inflammation and immunoregulatory function |
| EcN | G- | Maintaining intestinal flora homeostasis |
| Inflammatory bowel disease and ulcerative colitis therapy. | ||
| New immunomodulator development | ||
| Surface recombinant vaccine | ||
| New generation of drug delivery system through vesicle | ||
| B. fragilis | G- | A natural biological therapy for intestinal inflammatory diseases |
| Reinforcing immune homeostasis | ||
| B. vulgatus | G- | Immunomodulation and the preservation of a balanced gut microbiota |
| F. prausnitzii | G- | Anti-tumor effects or cancer therapy |
| Immunoregulator with anti-inflammation agents | ||
| L. vaginalis | G+ | Preventing HIV-1 transmission |
| L. sake | G+ | Enhancement of mucosal immune against infection |
| L. plantarum | G+ | Preventing scar formation |
| Anti-tumor effects in colonic cancer | ||
| Immunoregulator and anti-inflammation agents | ||
| Neurological recovery in ischemic stroke patients. | ||
| L. casei | G+ | Anti-infectious effects in intestine against pathogens |
| L. rhamnosus | G+ | Immunomodulator and anti-inflammation |
| Growth factor for injury treatment | ||
| A potential adjuvant anti-tumor agent in immunotherapy | ||
| C. butyricum | G+ | Immunomodulator and anti-inflammation |
| B. bifidum | G+ | Immunomodulator and anti-inflammation |
Table 1.
TOP
 MSK
MSK