Articles
- Page Path
- HOME > J. Microbiol > Volume 63(8); 2025 > Article
-
Full article
Mouse strain-dependent neutralizing antibody responses to Zika virus vaccines - Sang Hwan Seo, Jung-ah Choi, Eunji Yang, Hayan Park, Dae-Im Jung, Jae-Ouk Kim, Jae Seung Yang, Manki Song*
-
Journal of Microbiology 2025;63(8):e2504005.
DOI: https://doi.org/10.71150/jm.2504005
Published online: August 31, 2025
Science Unit, International Vaccine Institute, Seoul 08826, Republic of Korea
- *Correspondence Manki Song mksong@ivi.int
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
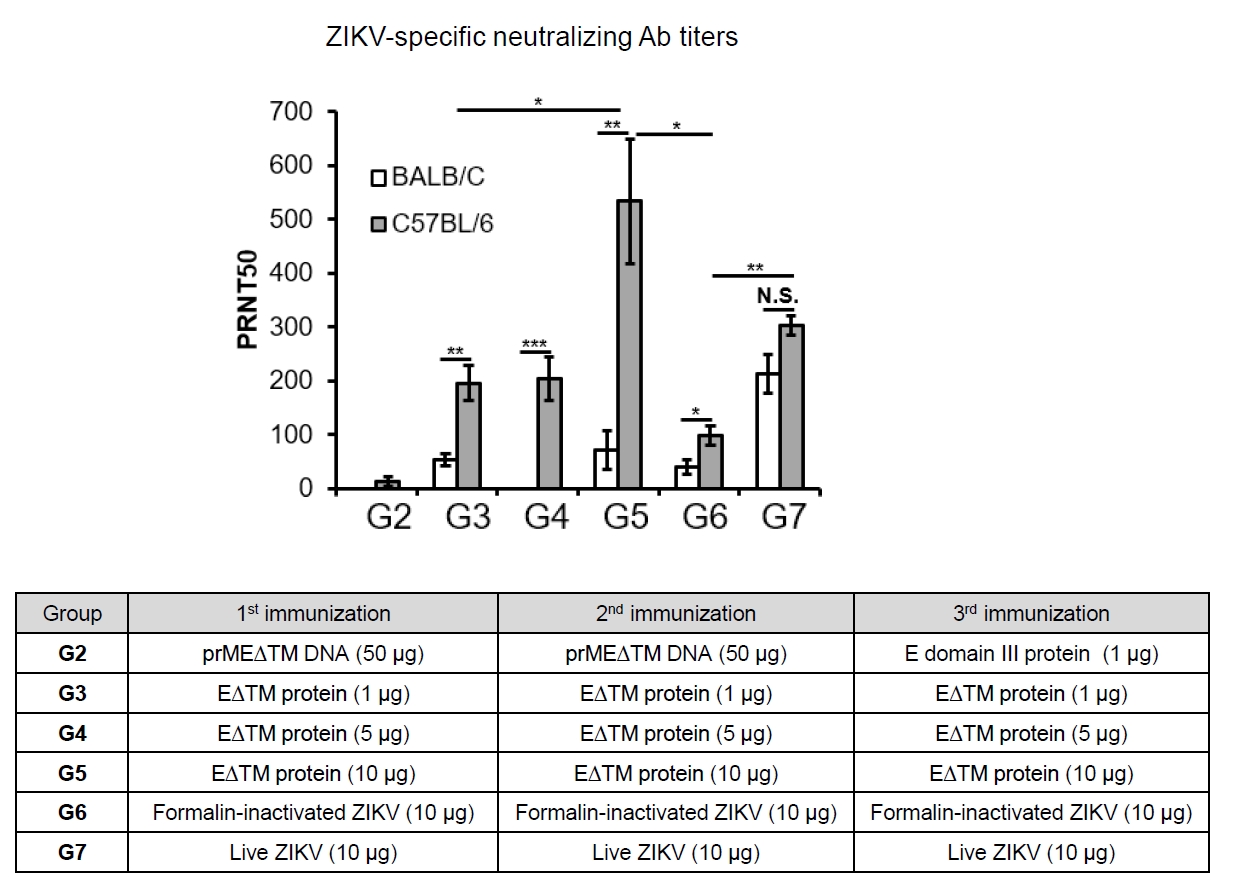
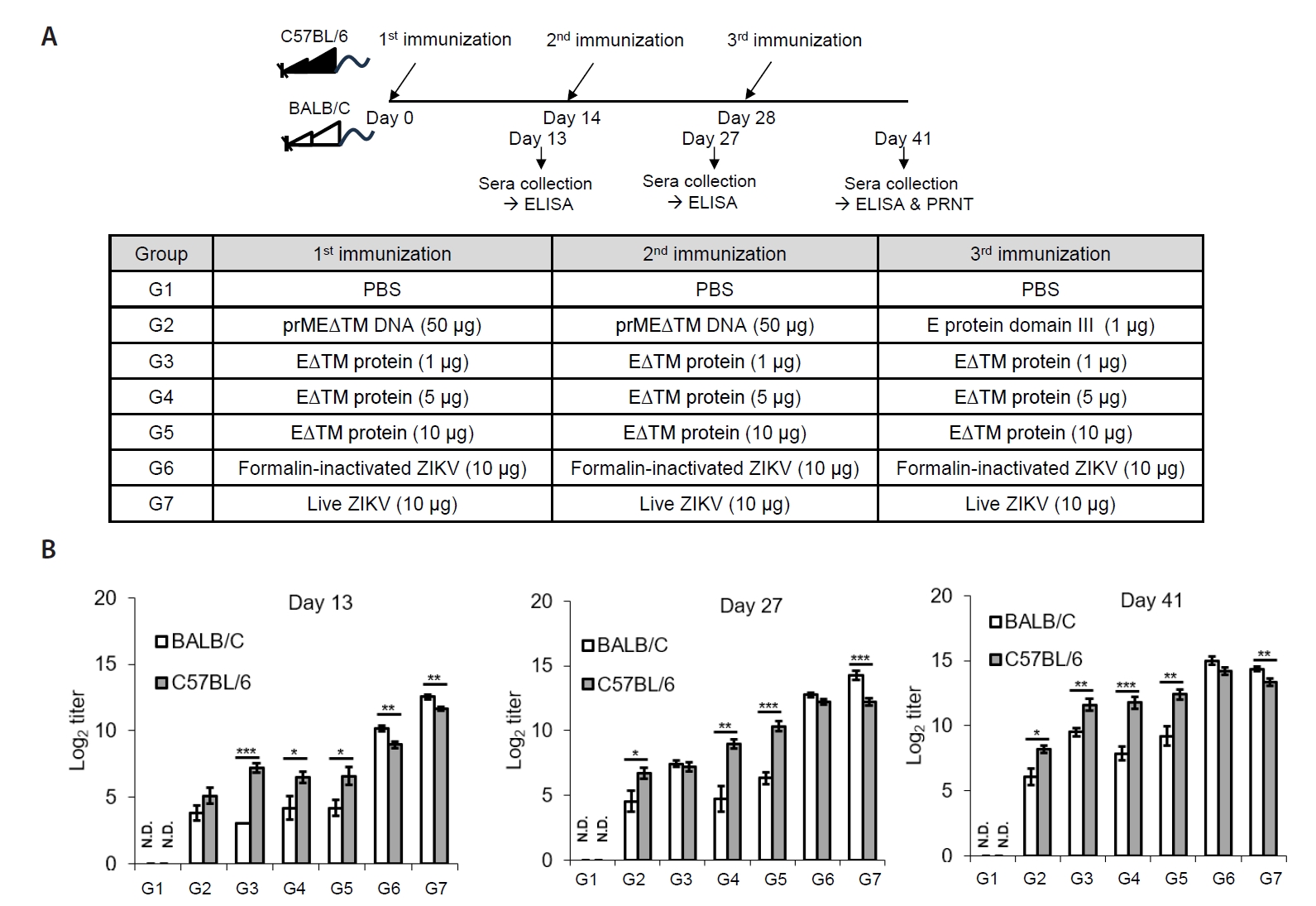
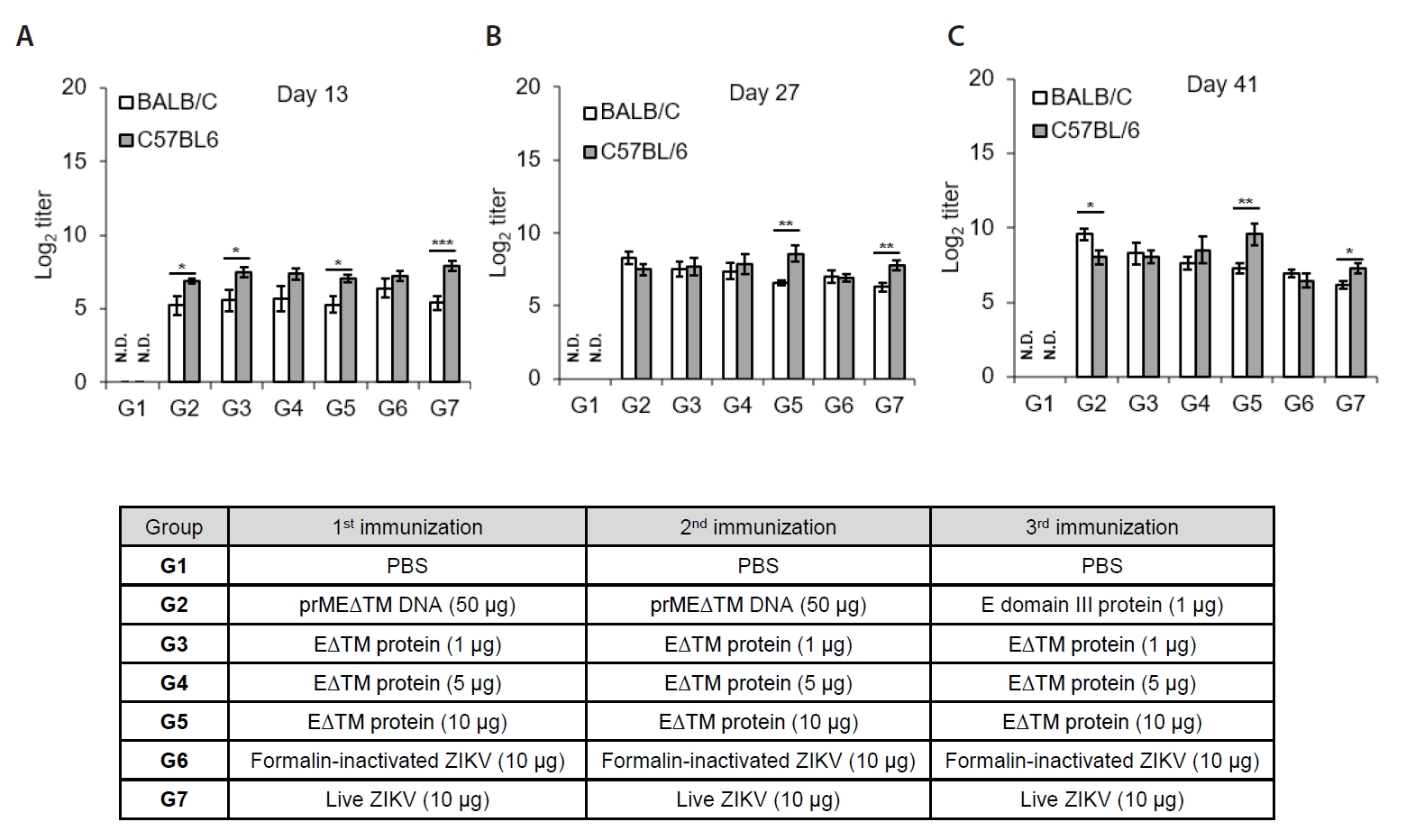
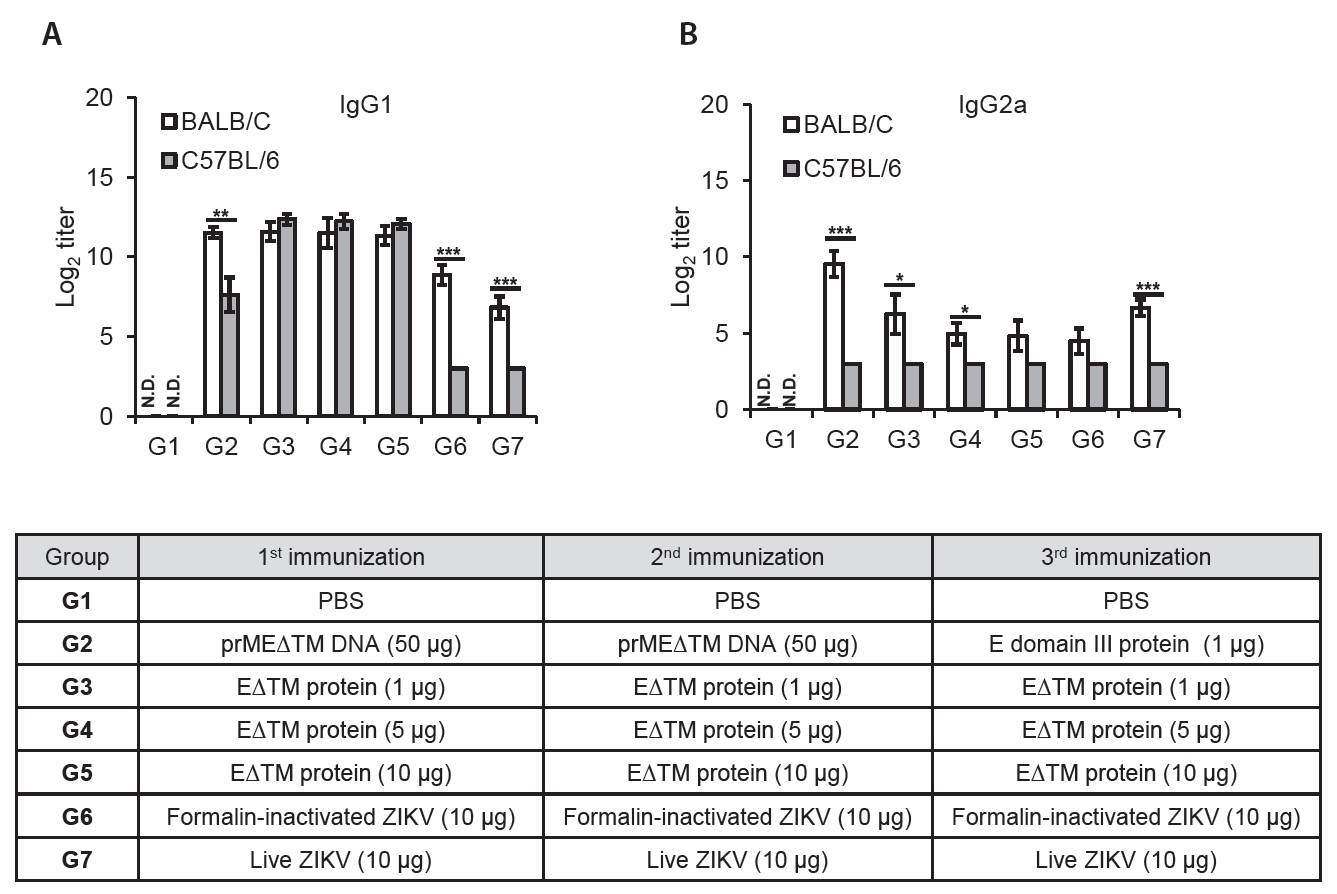
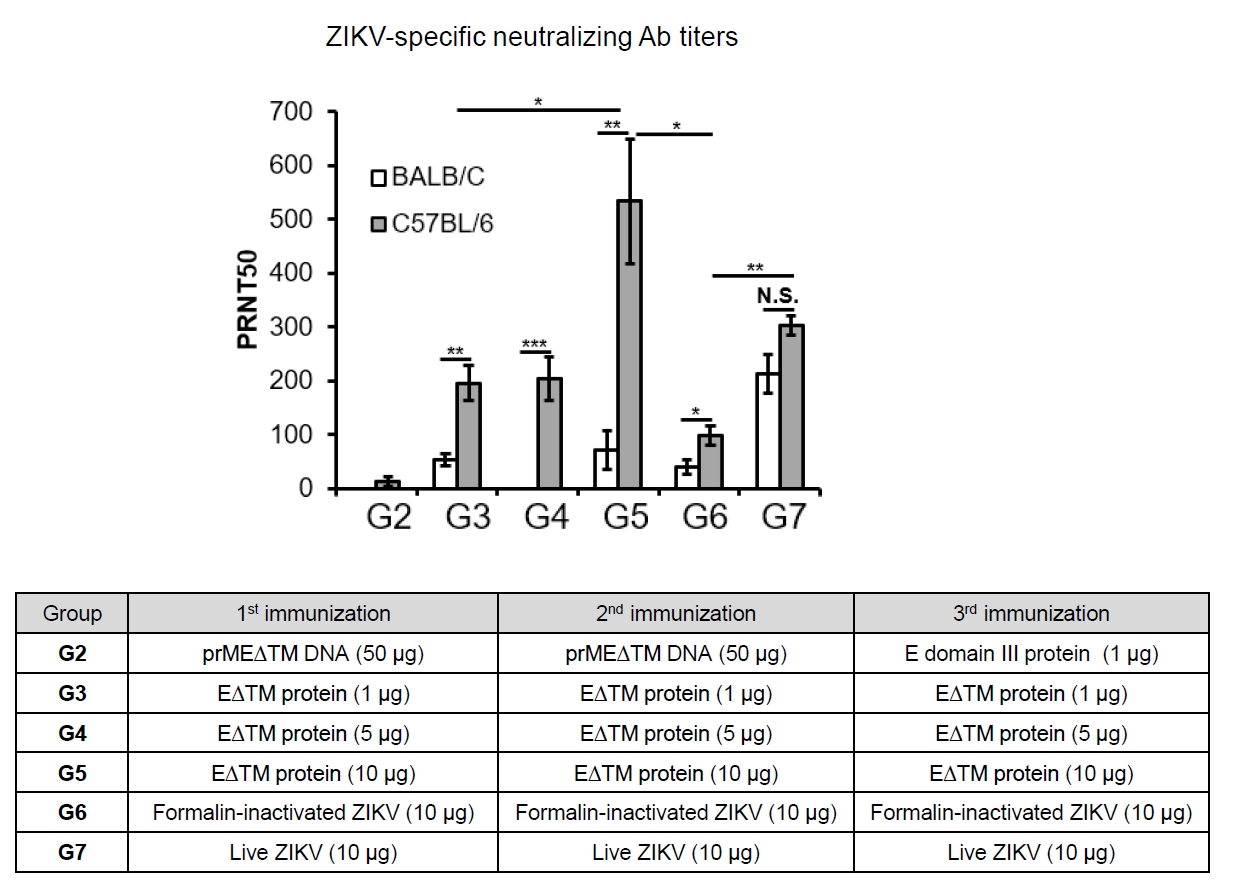
- The 2015 Zika virus (ZIKV) outbreak in Brazil and its global spread underscored the urgent need for effective and broadly protective vaccines. While C57BL/6 and BALB/c mice are widely used in preclinical vaccine research, direct comparisons of their ability to elicit ZIKV-specific neutralizing antibodies (nAbs) remain limited. This study aimed to systematically evaluate and compare the immunogenic potential of these two common mouse strains across diverse vaccine platforms, focusing on their capacity to generate functional neutralizing antibody responses. We assessed nAb and IgG responses following four vaccination strategies: (1) DNA vaccine encoding prMEΔTM followed by E protein domain III boost, (2) recombinant EΔTM protein expressed using baculovirus system, (3) formalin-inactivated ZIKV, and (4) live ZIKV. Although both strains generated detectable ZIKV- and E protein-specific IgG, the magnitude and quality of responses varied by vaccine platform and strain. Notably, C57BL/6 mice consistently mounted significantly higher nAb titers than BALB/c mice across all immunization groups, including subunit- and whole-virus-based vaccines. In contrast, BALB/c mice showed lower or undetectable nAb responses, despite comparable or higher total IgG levels in some cases. These findings show that host genetic background is a critical determinant of vaccine-induced neutralization and underscore the importance of selecting appropriate animal models in ZIKV vaccine development. C57BL/6 mice, due to their robust nAb responses, represent a reliable model for evaluating vaccine immunogenicity. Conversely, the limited nAb responses in BALB/c mice position them as a potential low-responder model, offering a stringent system to test the potency and breadth of protective immunity under suboptimal conditions.
Introduction
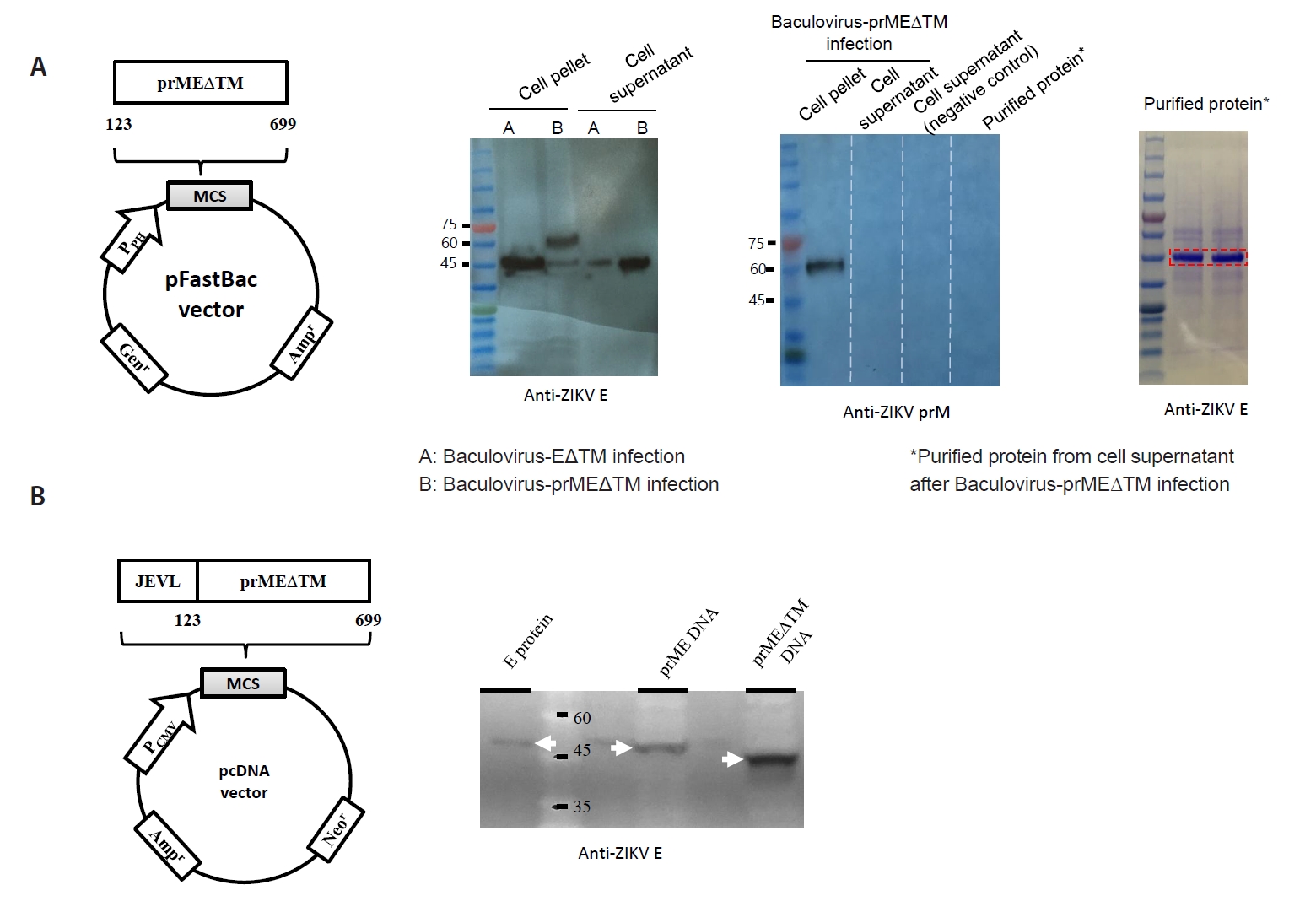
Materials and Methods
Results
Discussion
Acknowledgments
This work was funded by grants from the Korea Center for Disease Control and Prevention (2018-ER5502-00), Korea Ministry of Health and Welfare (HI15C2971), and Korea Ministry of Food and Drug Safety (grant 16172MFDS271). We thank Dr. Richard Jarman of WRAIR and Dr. Hyun-Soo Jo of Yonsei Univ. for kindly providing us with the ZIKV Brazil strain and pAcGP67 vector, respectively.
Conflict of Interest
The authors declare that they have no competing interests.
- Barrett ADT. 2018. Current status of Zika vaccine development: Zika vaccines advance into clinical evaluation. NPJ Vaccines. 3: 24.ArticlePubMedPMCPDF
- Chen YQ, Wohlbold TJ, Zheng NY, Huang M, Huang Y, et al. 2018. Influenza infection in humans induces broadly cross-reactive and protective neuraminidase-reactive antibodies. Cell. 173: 417–429. ArticlePubMedPMC
- Cibulski S, Varela APM, Teixeira TF, Cancela MP, Sesterheim P, et al. 2021. Zika virus envelope domain III recombinant protein delivered with saponin-based nanoadjuvant from Quillaja brasiliensis enhances anti-Zika immune responses, including neutralizing antibodies and splenocyte proliferation. Front Immunol. 12: 632714.ArticlePubMedPMC
- Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 29: 621–663. ArticlePubMed
- Dick GWA, Kitchen SF, Haddow AJ. 1952. Zika virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 46: 509–520. ArticlePubMed
- Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, et al. 2016. Rapid development of a DNA vaccine for Zika virus. Science. 354: 237–240. ArticlePubMedPMC
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, et al. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 360: 2536–2543. ArticlePubMed
- Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 453: 1122–1126. ArticlePubMedPMCPDF
- Essink B, Chu L, Seger W, Barranco E, Le Cam N, et al. 2023. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect Dis. 23: 621–633. ArticlePubMed
- Faria NR, Azevedo R, Kraemer MUG, Souza R, Cunha MS, et al. 2016. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 352: 345–349. ArticlePubMedPMC
- Fauci AS, Morens DM. 2016. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med. 374: 601–604. ArticlePubMed
- Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, et al. 2018. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet. 391: 552–562. ArticlePubMed
- Han HH, Diaz C, Acosta CJ, Liu M, Borkowski A. 2021. Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in healthy adults: an observer-blind, randomised, phase 1 trial. Lancet Infect Dis. 21: 1282–1292. ArticlePubMed
- Han K, Shao X, Zheng H, Wu C, Zhu J, et al. 2012. Revaccination of non- and low- responders after a standard three dose hepatitis B vaccine schedule. Hum Vaccin Immunother. 8: 1845–1849. ArticlePubMedPMC
- Hassert M, Wolf KJ, Schwetye KE, DiPaolo RJ, Brien JD, et al. 2018. CD4+ T cells mediate protection against Zika associated severe disease in a mouse model of infection. PLoS Pathog. 14: e1007237. ArticlePubMedPMC
- Hocart MJ, Mackenzie JS, Stewart GA. 1989. The immunoglobulin G subclass responses of mice to influenza A virus: The effect of mouse strain, and the neutralizing abilities of individual protein A-purified subclass antibodies. J Gen Virol. 70: 2439–2448. ArticlePubMed
- Iwasaki A, Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat Immunol. 16: 343–353. ArticlePubMedPMCPDF
- Krauer F, Riesen M, Reveiz L, Oladapo OT, Martinez-Vega R, et al. 2017. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barré syndrome: systematic review. PLoS Med. 14: e1002203. ArticlePubMedPMC
- Larocca RA, Abbink P, Peron JP, Zanotto PM, Iampietro MJ, et al. 2016. Vaccine protection against Zika virus from Brazil. Nature. 536: 474–478. ArticlePubMedPMCPDF
- Lazear HM, Diamond MS. 2016. Zika virus: New clinical syndromes and its emergence in the Western Hemisphere. J Virol. 90: 4864–4875. ArticlePubMedPMCLink
- Lee DK, Lee EY, Kim RH, Kwak HW, Kim JY, et al. 2018. Effect of apoptosis-associated speck-like protein containing a caspase recruitment domain on vaccine efficacy: Overcoming the effects of its deficiency with aluminum hydroxide adjuvant. Microbiol Immunol. 62: 176–186. ArticleLink
- Li H, Willingham SB, Ting JP, Re F. 2008. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 181: 17–21. ArticlePubMedPMCPDF
- Li X, Xu J, Lv Z, Wang J, Sun S, et al. 2015. Inadequate activation of the HBsAg-specific Th cells by APCs leads to hyporesponsiveness to HBsAg vaccine in B10.S mice. Hum Vaccin Immunother. 11: 1735–1743. ArticlePubMedPMC
- Lin HH, Yang SP, Tsai MJ, Lin GC, Wu HC, et al. 2019. Dengue and Zika virus domain III-flagellin fusion and glycan-masking E antigen for prime-boost immunization. Theranostics. 9: 4811–4826. ArticlePubMedPMC
- Lin HH, Yip BS, Huang LM, Wu SC. 2018. Zika virus structural biology and progress in vaccine development. Biotechnol Adv. 36: 47–53. ArticlePubMed
- Milich DR, Chen M, Schödel F, Peterson DL, Jones JE, et al. 1997. Role of B cells in antigen presentation of the hepatitis B core. Proc Natl Acad Sci USA. 94: 14648–14653. ArticlePubMedPMC
- Milich DR, Peterson DL, Schodel F, Jones JE, Hughes JL. 1995. Preferential recognition of hepatitis B nucleocapsid antigens by Th1 or Th2 cells is epitope and major histocompatibility complex dependent. J Virol. 69: 2776–2785. ArticlePubMedPMCLink
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 164: 6166–6173. ArticlePDF
- Modjarrad K, Lin L, George SL, Stephenson KE, Eckels KH, et al. 2018. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: Phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet. 391: 563–571. ArticlePubMed
- Ramos-Vara JA. 2005. Technical aspects of immunohistochemistry. Vet Pathol. 42: 405–426. ArticlePubMedLink
- Sanos SL, Kassub R, Testori M, Geiger M, Patzold J, et al. 2017. NLRC4 inflammasome-driven immunogenicity of a recombinant MVA mucosal vaccine encoding flagellin. Front Immunol. 8: 1988.ArticlePubMedPMC
- Sapparapu G, Fernandez E, Kose N, Bin C, Fox JM, et al. 2016. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 540: 443–447. ArticlePubMedPMCPDF
- Schenten D, Medzhitov R. 2011. Chaper 3 - The control of adaptive immune responses by the innate immune system. In Alt FW. (ed.), Advances in immunology, 109th vol, pp. 87-124. Academic Press.Article
- Snapper CM, Paul WE. 1987. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 236: 944–947. ArticlePubMed
- Sumathy K, Kulkarni B, Gondu RK, Ponnuru SK, Bonguram N, et al. 2017. Protective efficacy of Zika vaccine in AG129 mouse model. Sci Rep. 7: 46375.ArticlePubMedPMCPDF
- Suschak JJ, Wang S, Fitzgerald KA, Lu S. 2015. Identification of AIM2 as a sensor for DNA vaccines. J Immunol. 194: 630–636. ArticlePubMedPMCPDF
- Tebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, et al. 2017. Safety and immunogenicity of an anti-Zika virus DNA vaccine. N Engl J Med. 385: e35.ArticlePubMedPMC
- To A, Medina LO, Mfuh KO, Lieberman MM, Wong TAS, et al. 2018. Recombinant Zika virus subunits are immunogenic and efficacious in mice. mSphere. 3: e00576–17. ArticlePubMedPMCLink
- Wang W, Li G, De W, Luo Z, Pan P, et al. 2018. Zika virus infection induces host inflammatory responses by facilitating NLRP3 inflammasome assembly and interleukin-1β secretion. Nat Commun. 9: 106.ArticlePubMedPMCPDF
- Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, et al. 2013. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 501: 112–115. ArticlePubMedPMCPDF
- Woodson SE, Morabito KM. 2024. Continuing development of vaccines and monoclonal antibodies against Zika virus. NPJ Vaccines. 9: 91.ArticlePubMedPMCPDF
- Yang M, Lai H, Sun H, Chen Q. 2017. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci Rep. 7: 7679.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations

- The Pathogenesis and Virulence of the Major Enterovirus Pathogens Associated with Severe Clinical Manifestations: A Comprehensive Review
Yuwei Liu, Maiheliya Maisimu, Zhihang Ge, Suling Xiao, Haoran Wang
Cells.2025; 14(20): 1617. CrossRef - Development and Immunogenicity Assessment of a Multi-Epitope Antigen Against Zika Virus: An In Silico and In Vivo Approach
Lígia Rosa Sales Leal, Matheus Gardini Amâncio Marques de Sena, Maria da Conceição Viana Invenção, Ingrid Andrêssa de Moura, André Luiz Santos de Jesus, Georon Ferreira de Sousa, Bárbara Rafaela da Silva Barros, Cristiane Moutinho Lagos de Melo, Lindomar
Vaccines.2025; 14(1): 31. CrossRef
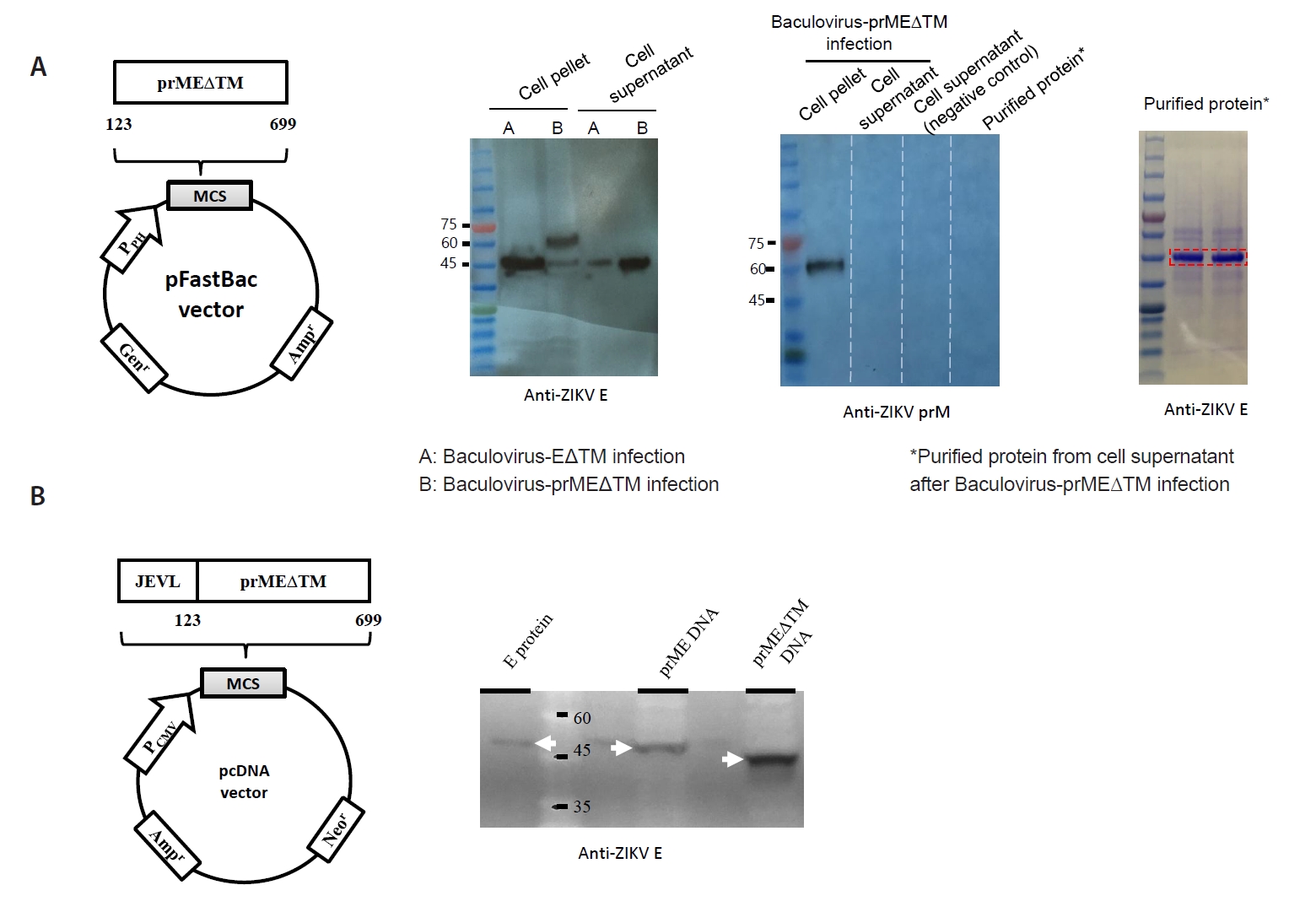
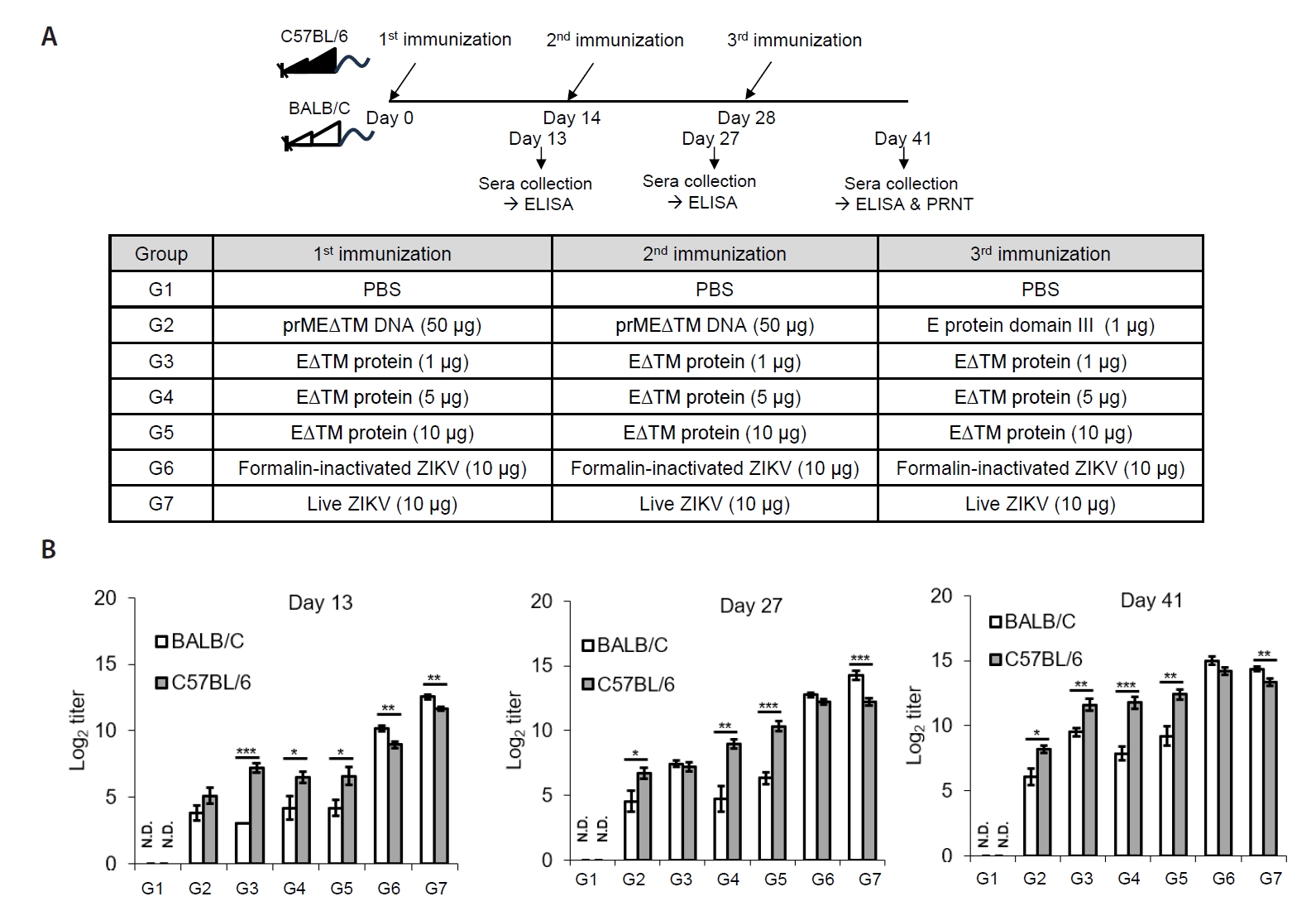
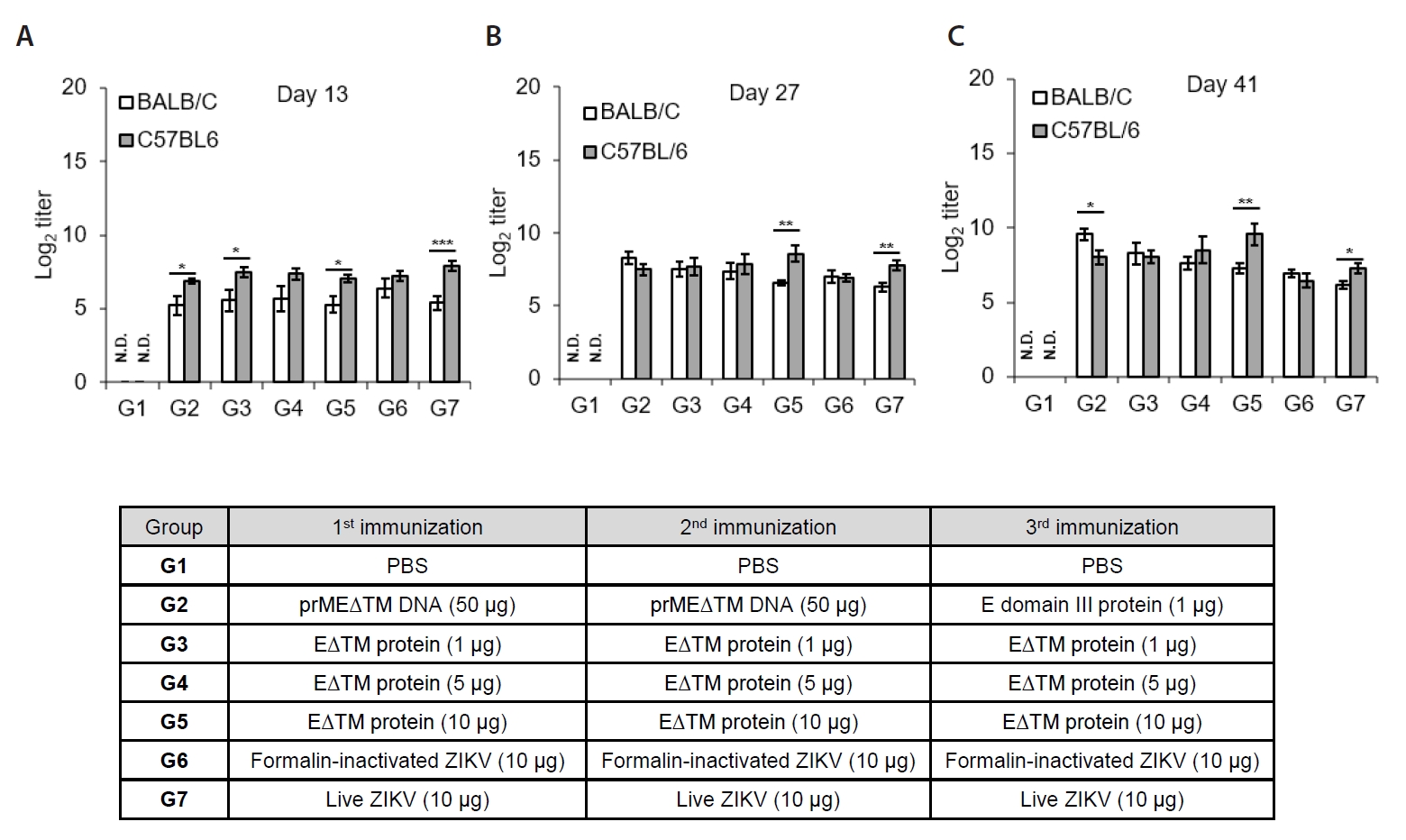
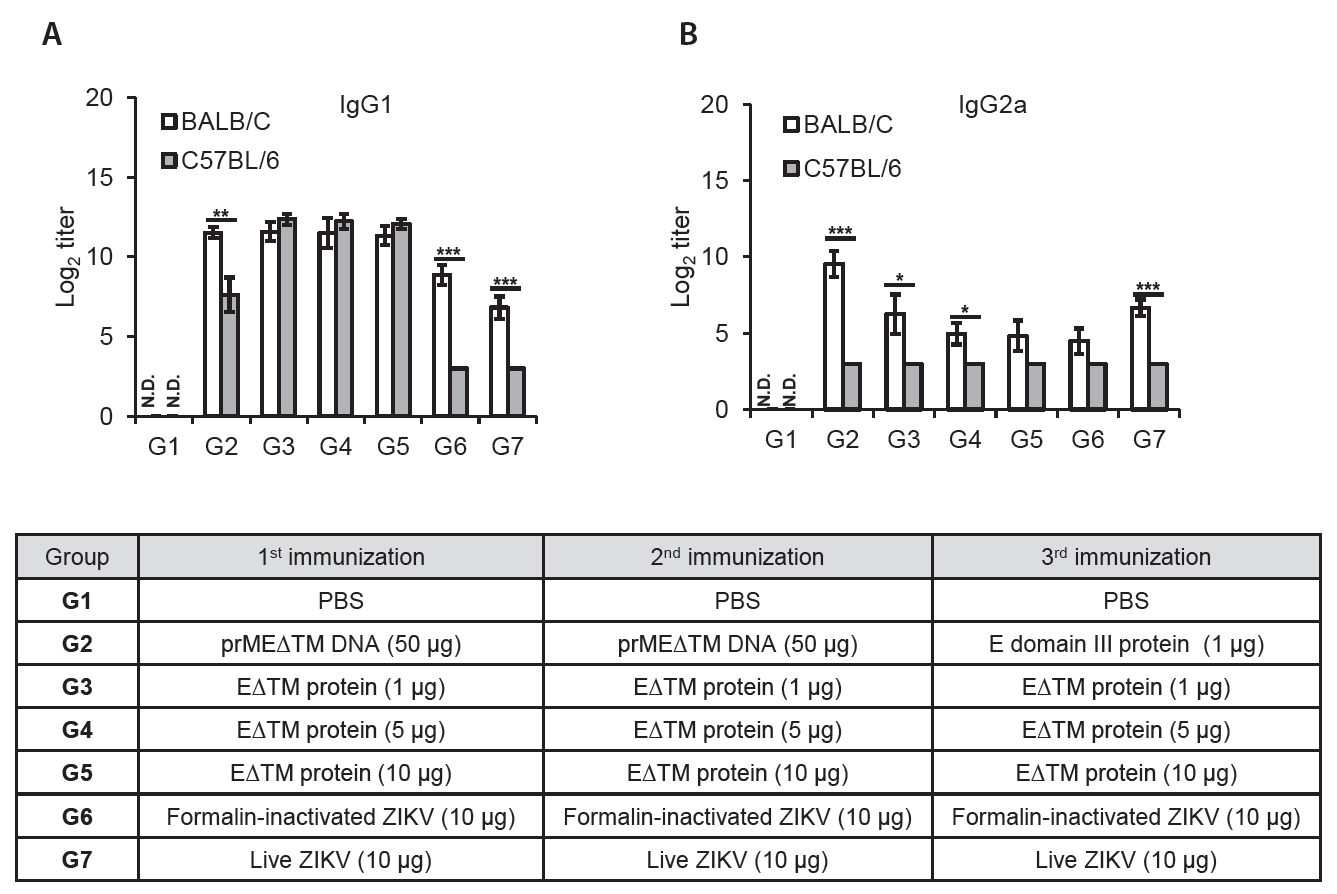
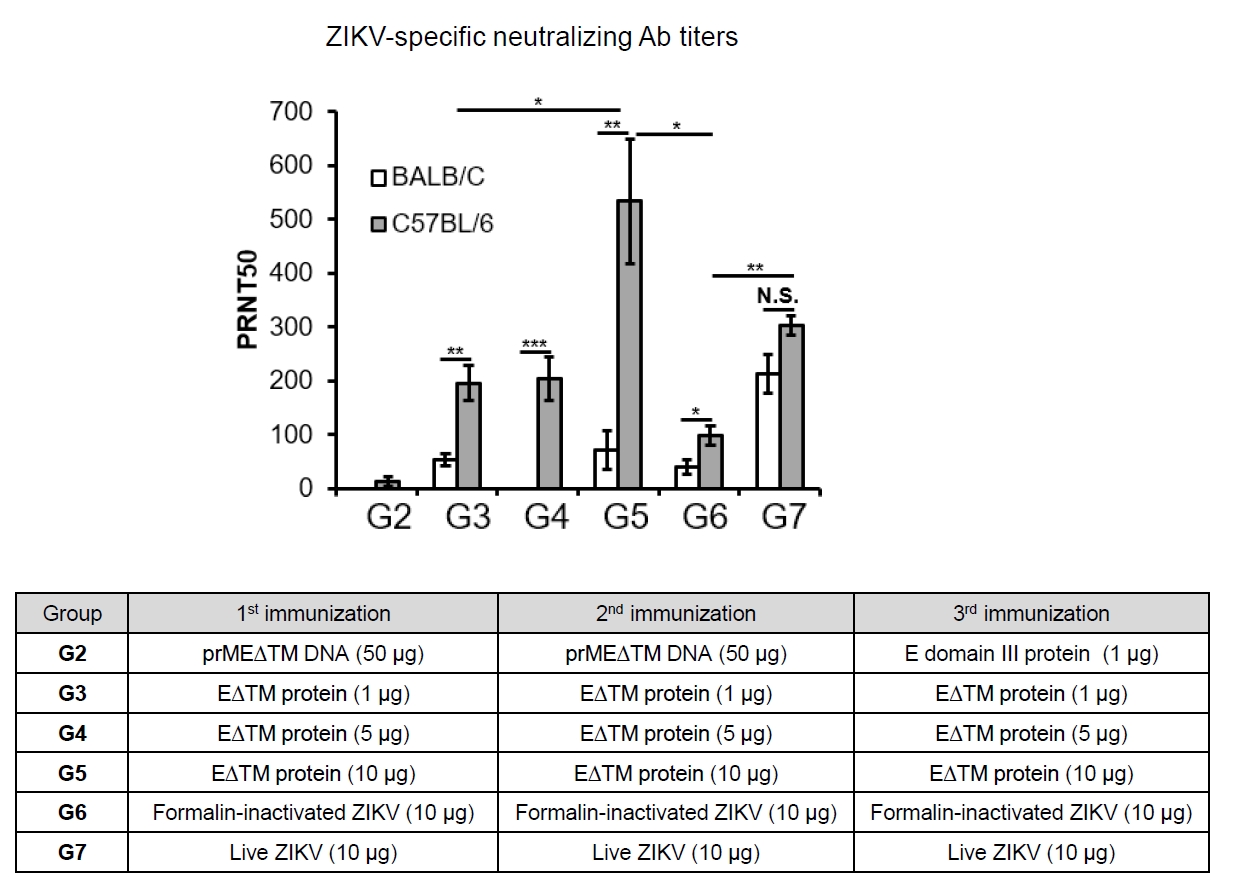
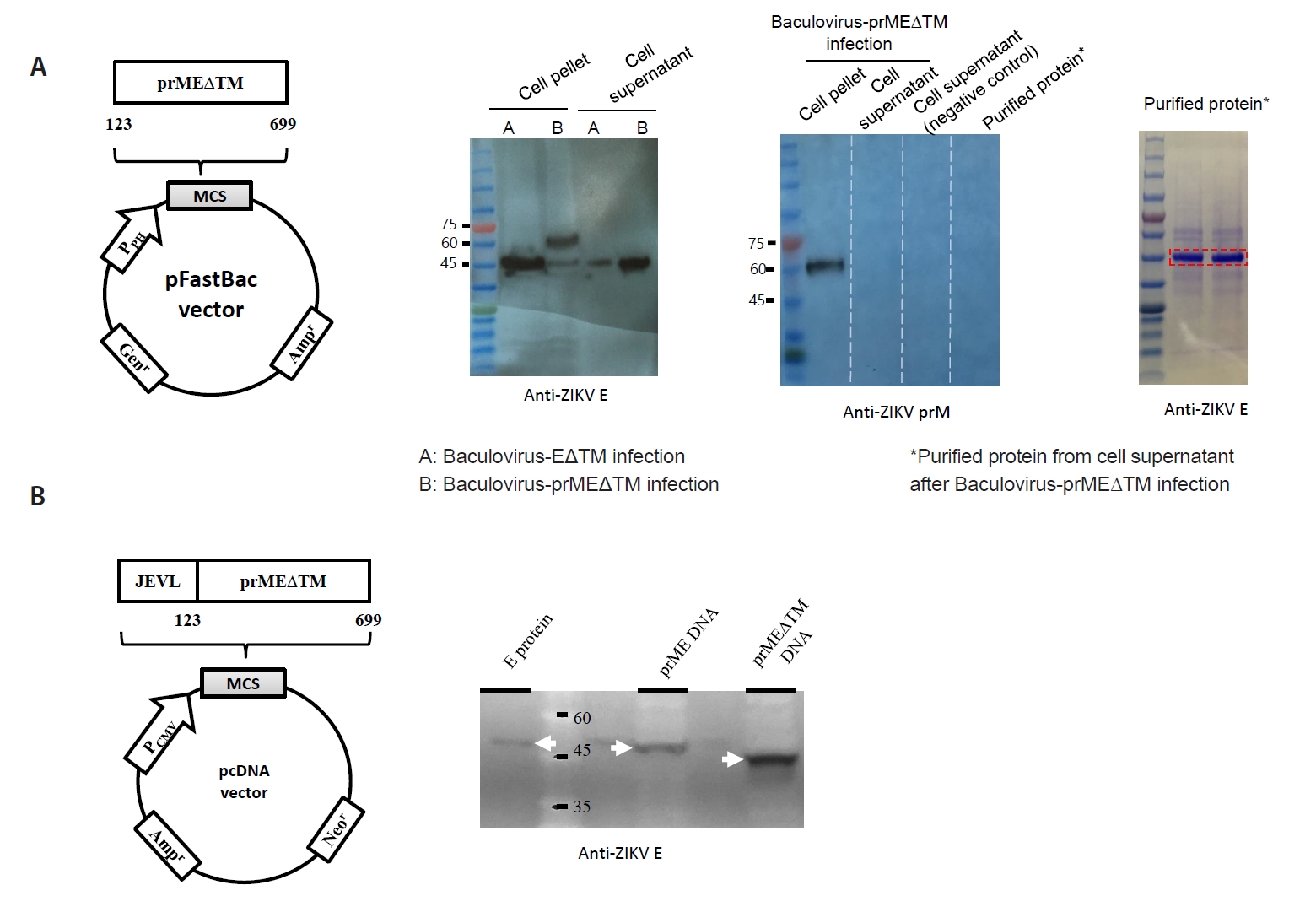
Fig. 1.
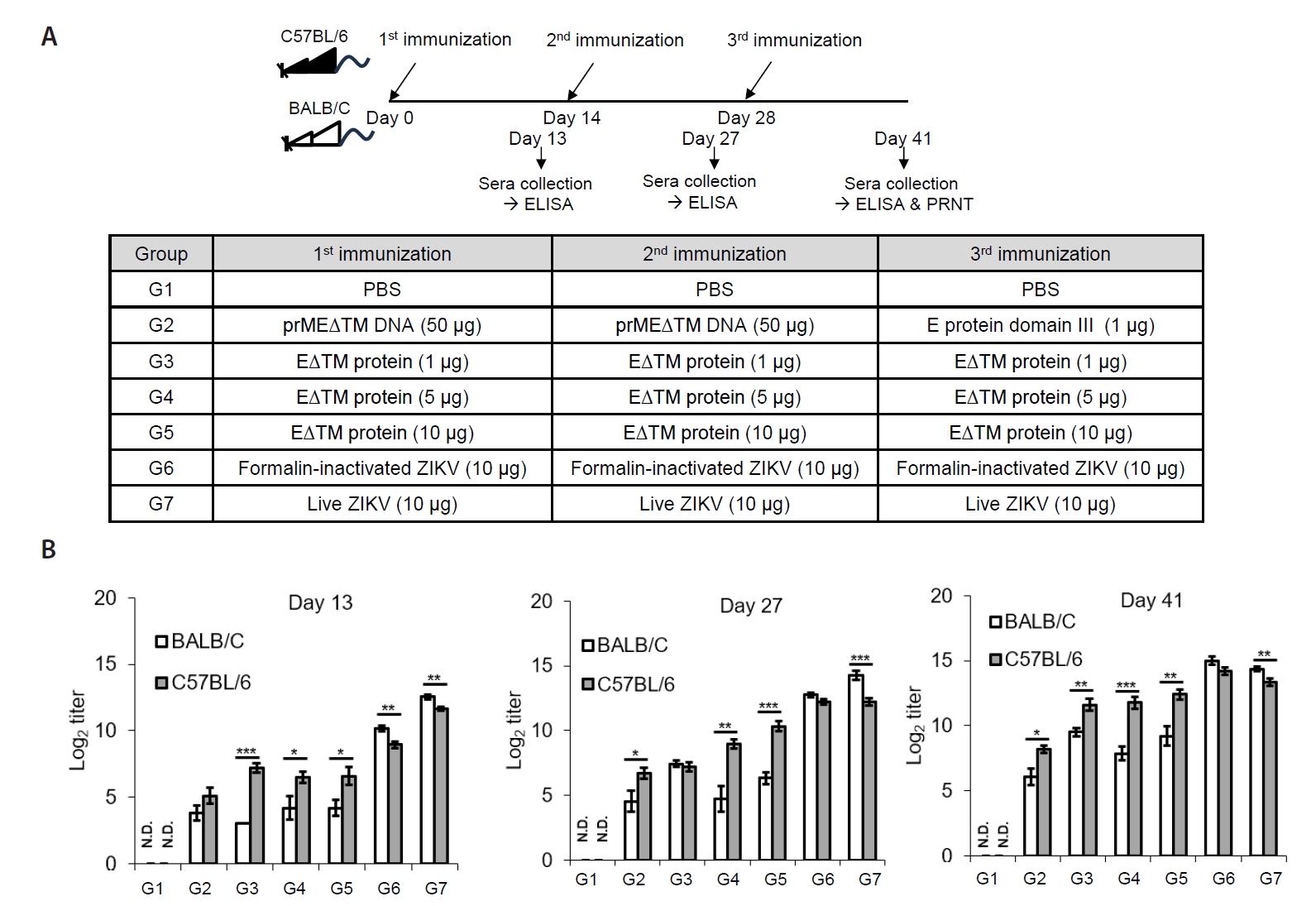
Fig. 2.
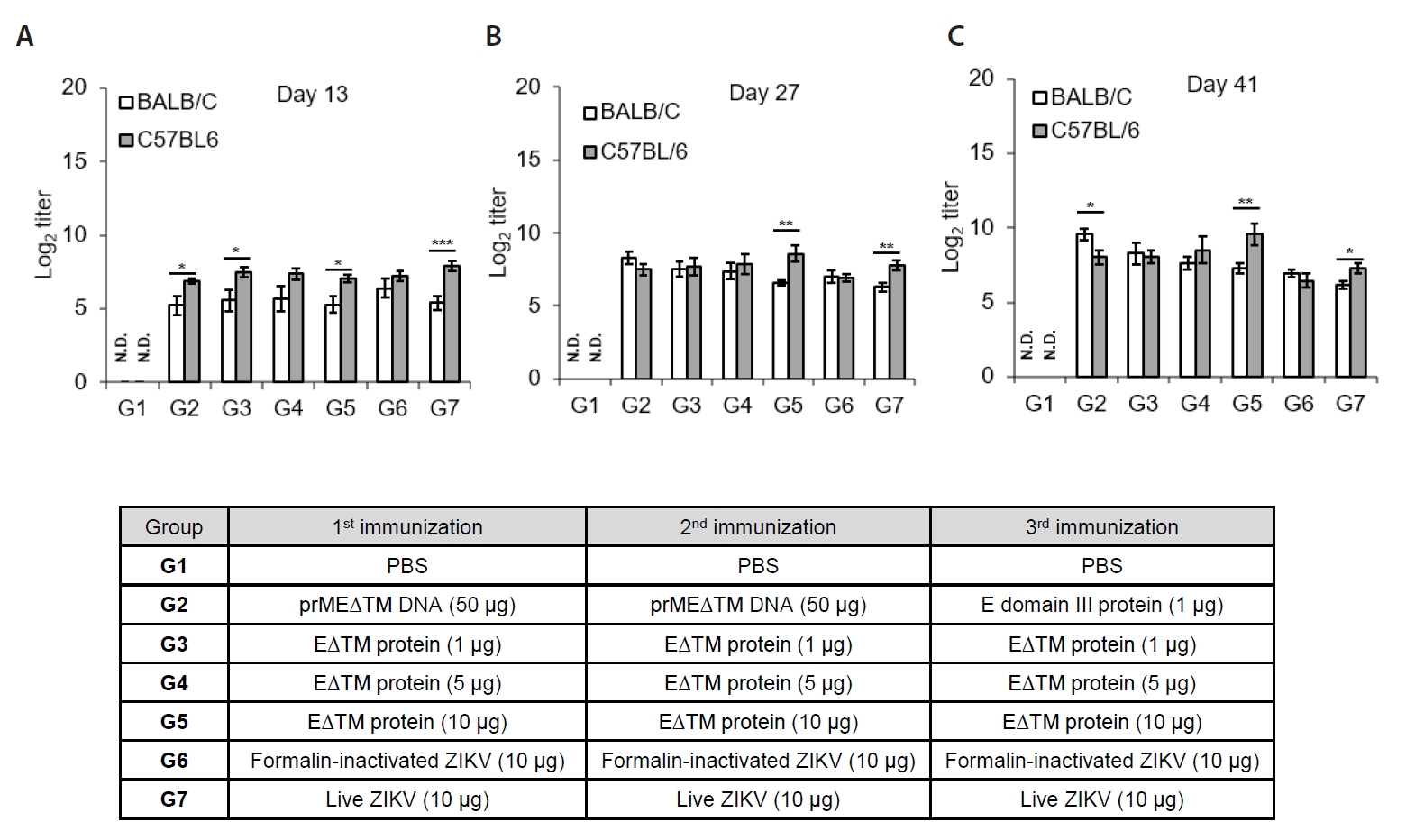
Fig. 3.
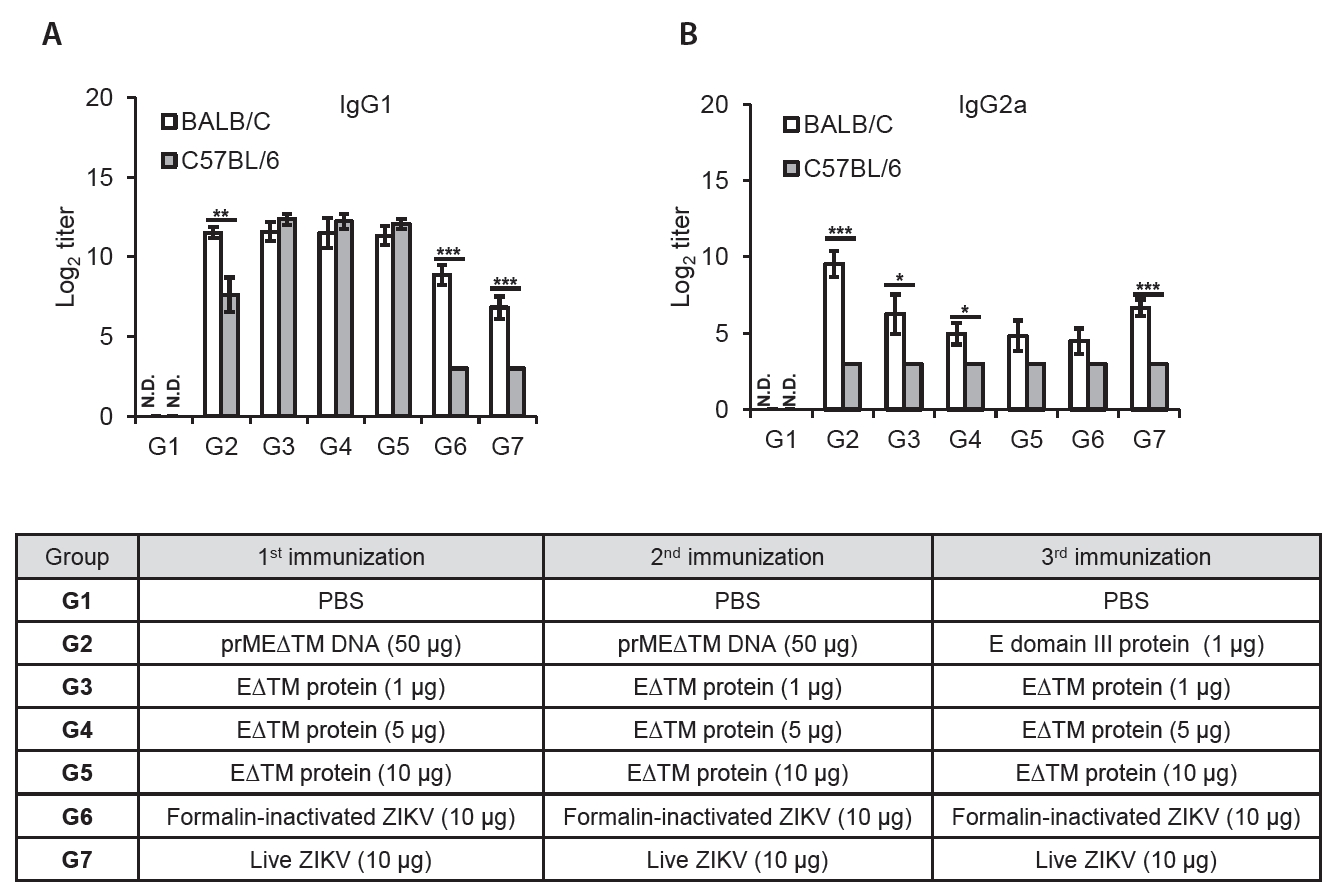
Fig. 4.
Fig. 5.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article