Articles
- Page Path
- HOME > J. Microbiol > Volume 63(7); 2025 > Article
-
Review
Metabolic engineering of Saccharomyces cerevisiae for efficient utilization of pectin-rich biomass - Dahye Lee1,2, Fransheska Semidey1,2, Luping Xu1,2, Eun Joong Oh1,2,*
-
Journal of Microbiology 2025;63(7):e2503001.
DOI: https://doi.org/10.71150/jm.2503001
Published online: July 31, 2025
1Department of Food Science, Purdue University, West Lafayette, IN 47907, USA
2Whistler Center for Carbohydrate Research, Purdue University, West Lafayette, IN 47907, USA
- *Correspondence Eun Joong Oh ejoh@purdue.edu
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 3,744 Views
- 109 Download
ABSTRACT
- Pectin-rich biomass, derived from fruit and citrus processing waste, presents a promising yet underutilized resource for sustainable biofuel and biochemical production. Its low lignin content and high concentrations of fermentable sugars, including D-galacturonic acid, L-arabinose, and D-xylose, make it an attractive feedstock. Unlike lignocellulosic biomass, pectin-rich hydrolysates require milder pretreatment, improving sugar recovery efficiency. However, industrial strains such as Saccharomyces cerevisiae exhibit strong glucose preference, limiting the efficient co-fermentation of mixed sugars. While prior reviews have broadly addressed lignocellulosic biomass utilization, this mini-review uniquely centers on the specific metabolic challenges and opportunities associated with pectin-rich feedstocks. In addition to incorporating established strategies for the co-utilization of cellobiose and xylose, we highlight recent advances that allow S. cerevisiae to metabolize carbon sources specifically from pectin-rich biomass, such as L-arabinose and D-galacturonic acid—monomers not prevalent in traditional lignocellulosic biomass. By integrating discussions on sugar transport engineering, redox balancing, and pathway optimization, this review offers a comprehensive framework to overcome glucose repression and support efficient co-fermentation of carbon sources from conventional and pectin-rich biomass. Drawing on these advances, we outline practical strategies to enhance fermentation performance and expand the valorization of food processing residues in biomanufacturing.
Introduction
Metabolic Pathways of Non-glucose Carbon Sources from Pectin-rich Biomass
Microbial Strategies for Cellulose utilization
Engineering Sugar Transporters for Enhanced Fermentation in S. cerevisiae
Metabolic Engineering of the Pentose Phosphate Pathway for Enhanced Pentose Utilization in S. cerevisiae
Regulatory Engineering for Improved Non-glucose Carbon Source Fermentation in S. cerevisiae
Enhancing Simultaneous Co-utilization of Mixed Sugars
Conclusion and Future Perspectives
Acknowledgments
This research was supported by startup funds from the College of Agriculture at Purdue University. Illustrations were created using BioRender.com.
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Statements
Not applicable.
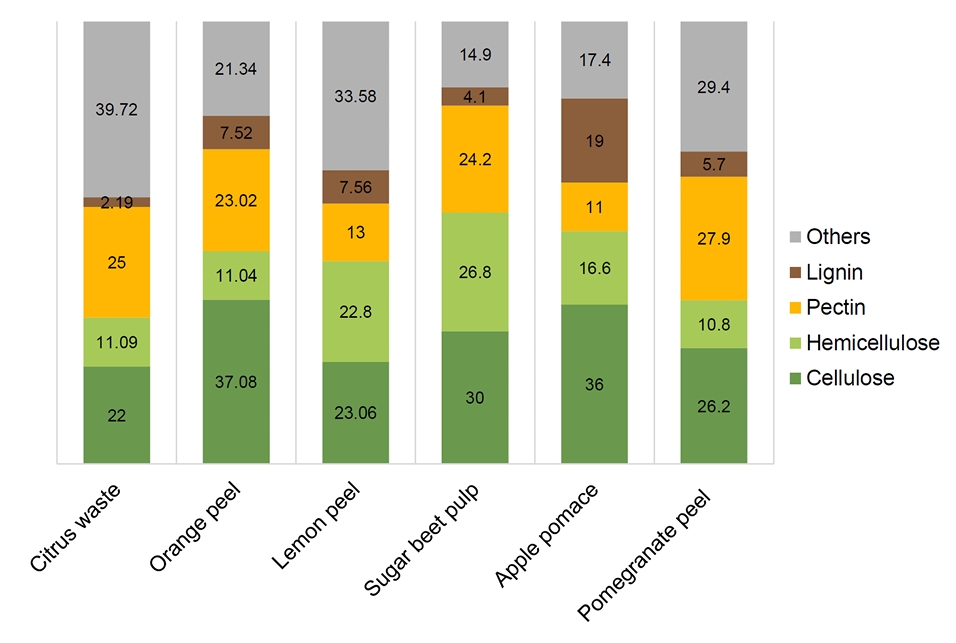
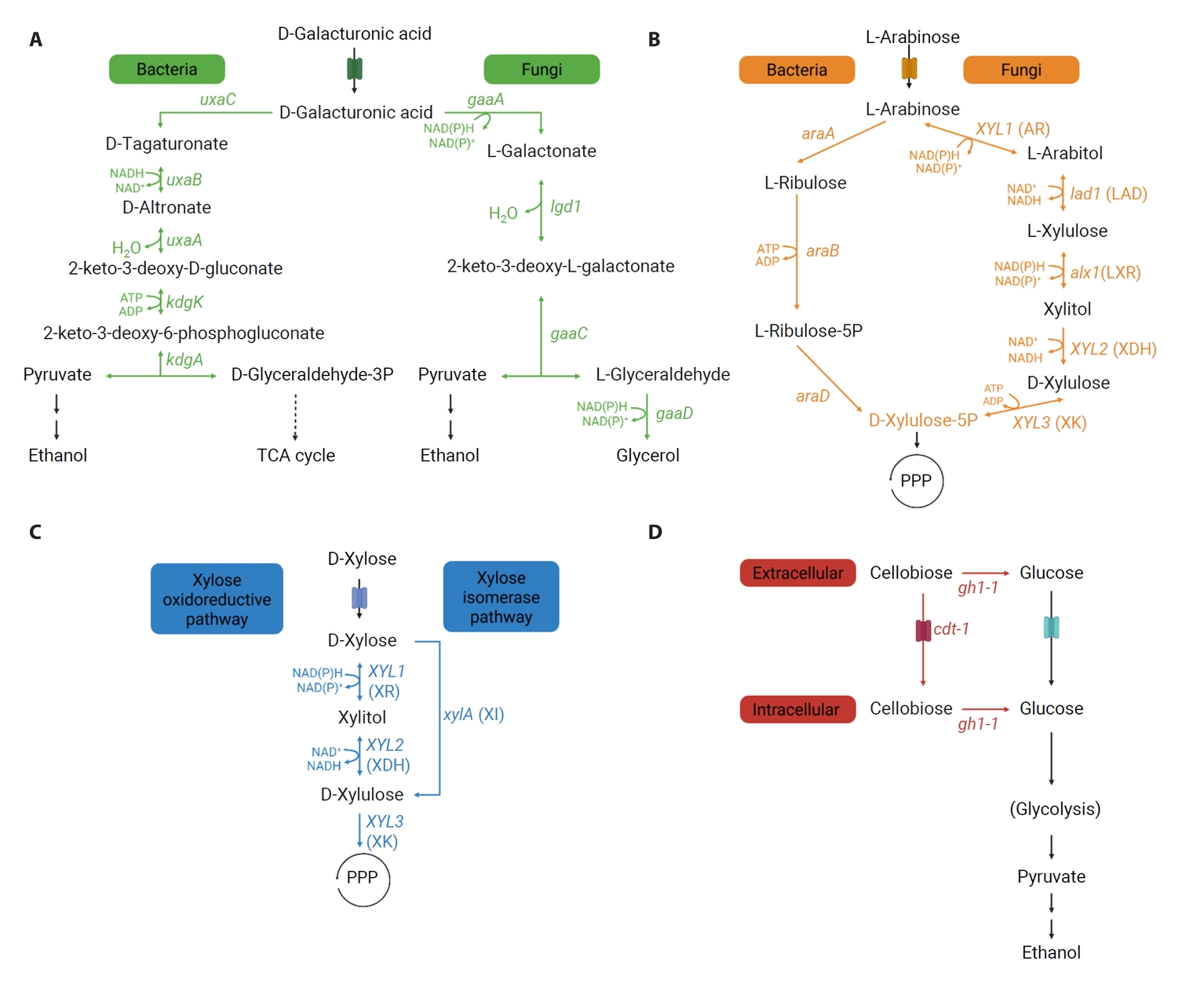
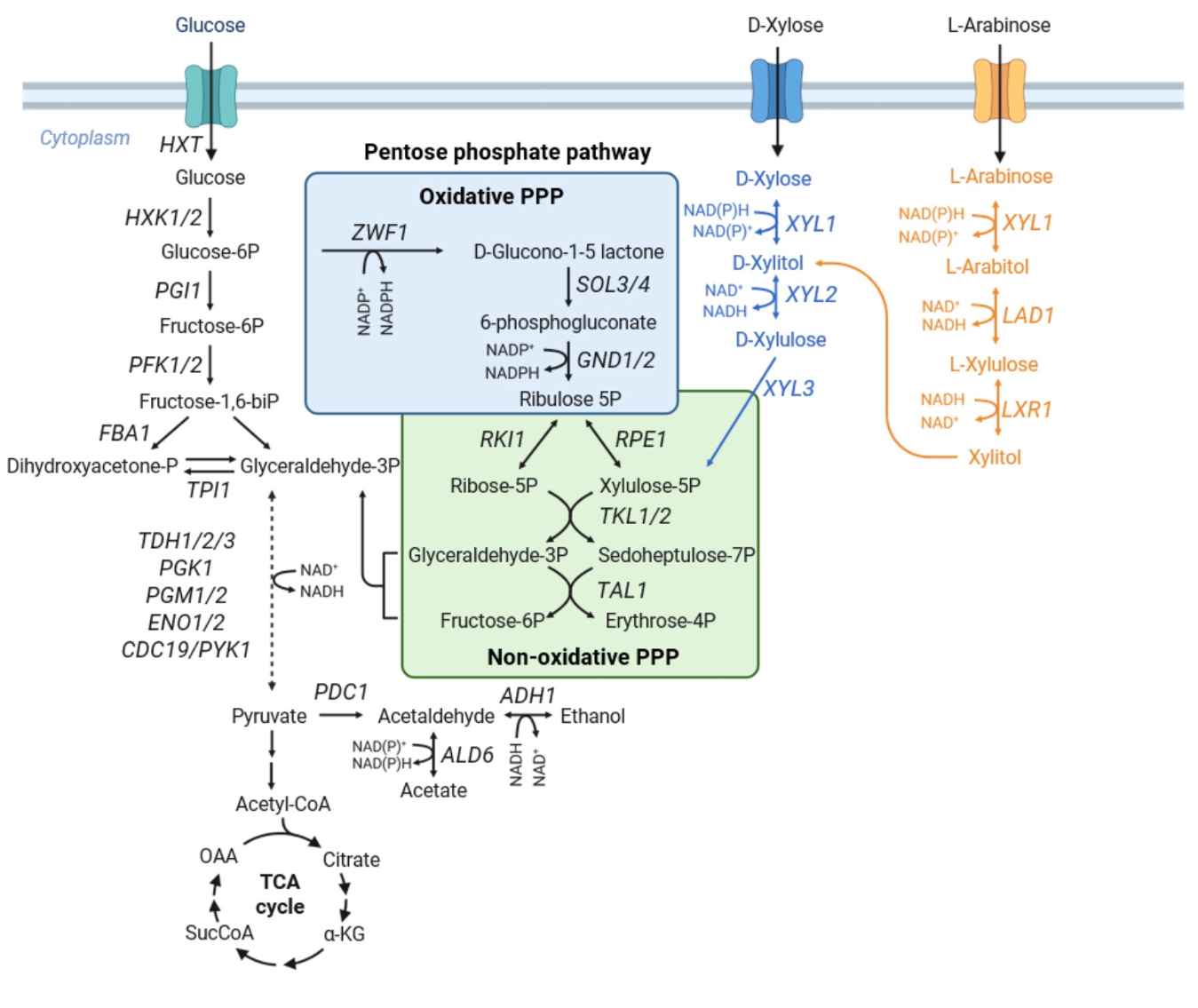
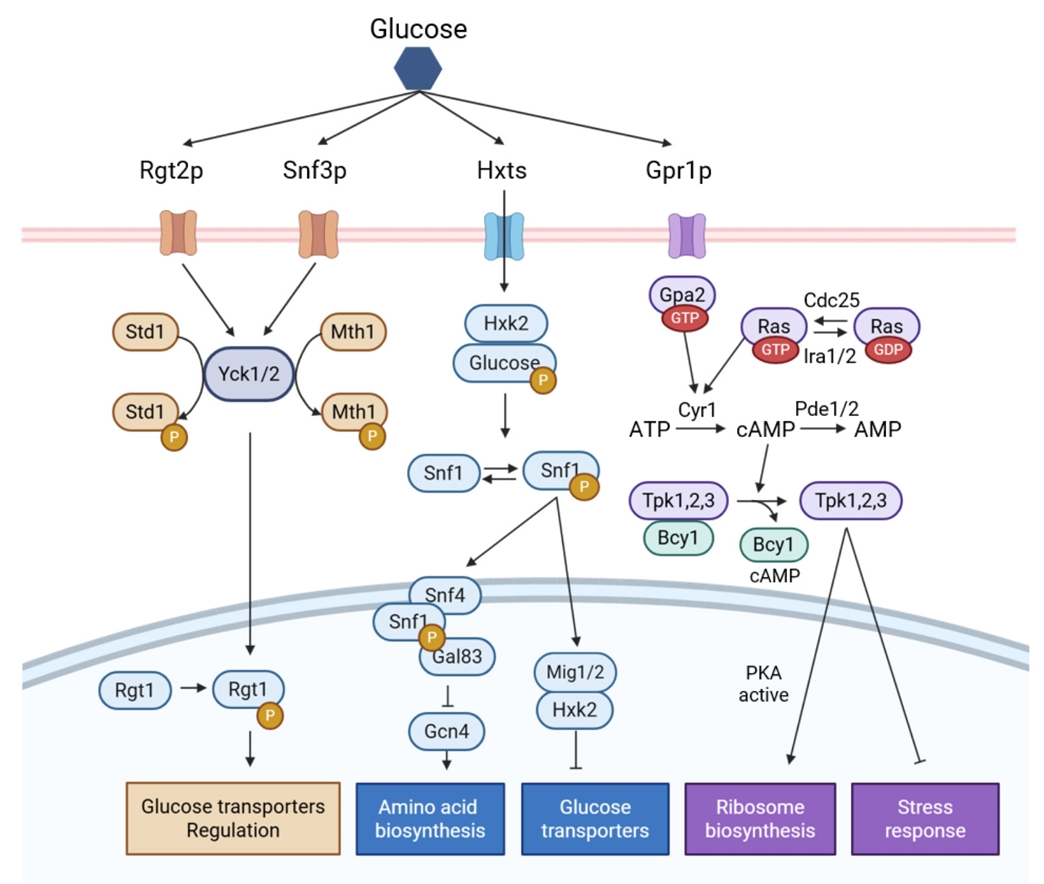
| Feedstock | Pretreatment | Hydrolysis | Glc | GalUA | Ara | Gal | Xyl | Rha | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Sugar beet pulp | Steam pretreatment at 5 bar pressure for 24 min | Enzymatic hydrolysis using cellulase 13L-C013L | 25.9 | 14.4 | 23 | 6.2 | 1.7 | 2.4 | Hamley-Bennett et al. (2016) |
| Apple pomace | Acid pretreatment, 72% sulfuric acid 16 min at 91°C | Viscozyme L and celluclast mixture for 100 h | 13.1 | 20.6 | 10.9 | 3.1 | - | - | Gama et al. (2015) |
| Mandarin peel | UV sterilization, freeze-dried | Polygalacturonase expressed by engineered, Pichia pastoris | 39.4 | 27.3 | 3.3 | 3.9 | 2.4 | 2.9 | Yang et al. (2020) |
| Orange peel | UV sterilization, freeze-dried | Polygalacturonase expressed by engineered, Pichia pastoris | 35.5 | 25.6 | 5.6 | 2.7 | 2.2 | 2.1 | Yang et al. (2020) |
| Jackfruit peel | Blanched, 80% (v/v) ethanol | 2 M H2SO4 110°C for 6 h | 3.5 | 57.0 | 5.2 | 23.3 | - | 9.7 | Li et al. (2019) |
| Melon peel | Microwave-assisted extraction with hexane and ethanol | Trifluoroacetic acid at 120°C for 75 min | 14.4 | 40.8 | 30.1 | 8.3 | - | 1.2 | Golbargi et al. (2021) |
| Passion fruit peel | Ultrasound-microwave-assisted three-phase partitioning | Trifluoroacetic acid at 110°C for 6 h | 8.3 | 56.6 | 25.3 | 7.9 | - | 9.0 | Zhao et al. (2023) |
| Watermelon rind | Freeze-dried, 96% (v/v) ethanol | 2 M HCl in dry methanol for 5 h at 100°C | 57.1 | 26.9 | 2.8 | 17.9 | 6.2 | 1.5 | Mendez et al. (2021) |
| Pectin-rich residue | Fermentation type | Hydrolysis | Fermentable sugars1) (g/L) | pH | Ethanol yield (g/g) | Productivity (g/L/h) | Time (h) | Reference |
|---|---|---|---|---|---|---|---|---|
| Orange peel citrus waste | SSF | Pectinase, cellulase, β-glucosidase | 5.93 | 6 | 0.67 | 1.50 | 24 | Wilkins et al. (2007) |
| Orange peel citrus waste | SSF | Pectinase, cellulase, β-glucosidase | - | 4.2–4.8 | 4.05% (w/v) | - | 18 | Zhou et al. (2008) |
| Mango peel hydrolysate | SHF | Pectinase, trizyme 50 | 150 | 5 | 0.476 | 0.99 | 72 | Reddy et al. (2011) |
| Mandarin peel | SHF | In-house from enzyme HEA and HEB | 68.5 | 4.8 | 0.43 | 3.28 | 9 | Choi et al. (2015) |
| Grapefruit peel | SHF | In-house from enzyme HEA and HEB | 51.8 | 4.8 | 0.42 | 2.40 | 9 | Choi et al. (2015) |
| Lemon peel | SHF | In-house from enzyme HEA and HEB | 46.8 | 4.8 | 0.42 | 2.18 | 9 | Choi et al. (2015) |
| Lime peel | SHF | In-house from enzyme HEA and HEB | 34.8 | 4.8 | 0.41 | 1.60 | 9 | Choi et al. (2015) |
| Orange peel | CBP | Genome integration of the cellulase gene from Ampullaria gigas Spix | 20.0 | - | 0.38 | 0.16 | 48 | Yang et al. (2018) |
| Orange processing waste | SSF | Pectinase, cellulase | 57.7 | 4.2 | 0.48 | 0.56 | 48 | Widmer et al. (2010) |
| Sweet lime peel | SHF | Pectinase, cellulase | 7.10 | - | 0.092 | - | 72 | John et al. (2017) |
| Citrus peel waste | SHF | Acid hydrolysis | 101 | 4.8 | 0.63 | 3.42 | 9 | Kyriakou et al. (2020) |
| Orange citrus waste | SHF | Acid hydrolysis | 29.0 | 5 | 0.43 | 1.65 | 24 | Pourbafrani et al. (2010) |
- Ahmad Khorairi ANS, Sofian-Seng NS, Othaman R, Abdul Rahman H, Mohd Razali NS, et al. 2023. A review on agro-industrial waste as cellulose and nanocellulose source and their potentials in food applications. Food Rev Int. 39: 663–688. Article
- Becker J, Boles E. 2003. A modified Saccharomyces cerevisiae strain that consumes L-arabinose and produces ethanol. Appl Environ Microbiol. 69: 4144–4150. ArticlePubMedPMCLink
- Benz JP, Protzko RJ, Andrich JM, Bauer S, Dueber JE, et al. 2014. Identification and characterization of a galacturonic acid transporter from Neurospora crassa and its application for Saccharomyces cerevisiae fermentation processes. Biotechnol Biofuels. 7: 20.ArticlePubMedPMC
- Bera AK, Sedlak M, Khan A, Ho NW. 2010. Establishment of L-arabinose fermentation in glucose/xylose co-fermenting recombinant Saccharomyces cerevisiae 424A(LNH-ST) by genetic engineering. Appl Microbiol Biotechnol. 87: 1803–1811. ArticlePubMedPDF
- Bettiga M, Bengtsson O, Hahn-Hägerdal B, Gorwa-Grauslund MF. 2009. Arabinose and xylose fermentation by recombinant Saccharomyces cerevisiae expressing a fungal pentose utilization pathway. Microb Cell Fact. 8: 40.ArticlePubMedPMC
- Biz A, Sugai-Guerios MH, Kuivanen J, Maaheimo H, Krieger N, et al. 2016. The introduction of the fungal D-galacturonate pathway enables the consumption of D-galacturonic acid by Saccharomyces cerevisiae. Microb Cell Fact. 15: 144.ArticlePubMedPMC
- Bracher JM, Verhoeven MD, Wisselink HW, Crimi B, Nijland JG, et al. 2018. The Penicillium chrysogenum transporter PcAraT enables high-affinity, glucose-insensitive L-arabinose transport in Saccharomyces cerevisiae. Biotechnol Biofuels. 11: 63.ArticlePubMedPMCPDF
- Brink DP, Borgstrom C, Tueros FG, Gorwa-Grauslund MF. 2016. Real-time monitoring of the sugar sensing in Saccharomyces cerevisiae indicates endogenous mechanisms for xylose signaling. Microb Cell Fact. 15: 183.ArticlePubMedPMCPDF
- Cai Y, Qi X, Qi Q, Lin Y, Wang Z, et al. 2018. Effect of MIG1 and SNF1 deletion on simultaneous utilization of glucose and xylose by Saccharomyces cerevisiae. Sheng Wu Gong Cheng Xue Bao. 34: 54–67. ArticlePubMed
- Cakmak H, Dekker M. 2022. Optimization of cellulosic fiber extraction from parsley stalks and utilization as filler in composite biobased films. Foods. 11: 3932.ArticlePubMedPMC
- Canelas AB, van Gulik WM, Heijnen JJ. 2008. Determination of the cytosolic free NAD/NADH ratio in Saccharomyces cerevisiae under steady-state and highly dynamic conditions. Biotechnol Bioeng. 100: 734–743. ArticlePubMed
- Chauve M, Mathis H, Huc D, Casanave D, Monot F, et al. 2010. Comparative kinetic analysis of two fungal beta-glucosidases. Biotechnol Biofuels. 3: 3.ArticlePubMedPMC
- Chen B, Lee HL, Heng YC, Chua N, Teo WS, et al. 2018. Synthetic biology toolkits and applications in Saccharomyces cerevisiae. Biotechnol Adv. 36: 1870–1881. ArticlePubMed
- Choi IS, Lee YG, Khanal SK, Park BJ, Bae HJ. 2015. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl Energy. 140: 65–74. Article
- Das H, Singh SK. 2004. Useful byproducts from cellulosic wastes of agriculture and food industry—a critical appraisal. Crit Rev Food Sci Nutr. 44: 77–89. ArticlePubMed
- dos Reis TF, de Lima PBA, Parachin NS, Mingossi FB, de Castro Oliveira JV, et al. 2016. Identification and characterization of putative xylose and cellobiose transporters in Aspergillus nidulans. Biotechnol Biofuels. 9: 204.ArticlePubMedPMC
- Edwards MC, Doran-Peterson J. 2012. Pectin-rich biomass as feedstock for fuel ethanol production. Appl Microbiol Biotechnol. 95: 565–575. ArticlePubMedPMC
- Eriksen DT, Hsieh PCH, Lynn P, Zhao HM. 2013. Directed evolution of a cellobiose utilization pathway in Saccharomyces cerevisiae by simultaneously engineering multiple proteins. Microb Cell Fact. 12: 61.ArticlePubMedPMCPDF
- Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 3: 289–306. ArticlePubMedPMC
- Fonseca C, Romao R, Rodrigues de Sousa H, Hahn-Hägerdal B, Spencer-Martins I. 2007. L-Arabinose transport and catabolism in yeast. FEBS J. 274: 3589–3600. ArticlePubMed
- Galazka JM, Tian C, Beeson WT, Martinez B, Glass NL, et al. 2010. Cellodextrin transport in yeast for improved biofuel production. Science. 330: 84–86. ArticlePubMed
- Gama R, Van Dyk JS, Pletschke BI. 2015. Optimisation of enzymatic hydrolysis of apple pomace for production of biofuel and biorefinery chemicals using commercial enzymes. 3 Biotech. 5: 1075–1087. ArticlePubMedPMCPDF
- Gao L, Liu G, Zhao Q, Xiao Z, Sun W, et al. 2022. Customized optimization of lignocellulolytic enzyme cocktails for efficient conversion of pectin-rich biomass residues. Carbohydr Polym. 297: 120025.ArticlePubMed
- Garnett MT, Kumar HKS, Beckingham BS, Alexander SL. 2024. Extraction of cellulose from restaurant food waste. RSC Sustainability. 2: 170–178. Article
- Golbargi F, Gharibzahedi SMT, Zoghi A, Mohammadi M, Hashemifesharaki R. 2021. Microwave-assisted extraction of arabinan-rich pectic polysaccharides from melon peels: Optimization, purification, bioactivity, and techno-functionality. Carbohydr Polym. 256: 117522.ArticlePubMed
- Gunders D, Bloom J. 2017. Wasted: How America is losing up to 40 percent of its food from farm to fork to landfill.Link
- Ha SJ, Galazka JM, Kim SR, Choi J-H, Yang X, et al. 2011a. Engineered Saccharomyces cerevisiae capable of simultaneous cellobiose and xylose fermentation. Proc Natl Acad Sci USA. 108: 504–509. ArticlePubMedPMC
- Ha SJ, Kim H, Lin YP, Jang MU, Galazka JM, et al. 2013. Single amino acid substitutions in HXT2.4 from Scheffersomyces stipitis lead to improved cellobiose fermentation by engineered Saccharomyces cerevisiae. Appl Environ Microbiol. 79: 1500–1507. ArticlePubMedPMCLink
- Ha SJ, Wei Q, Kim SR, Galazka JM, Cate JHD, et al. 2011b. Cofermentation of cellobiose and galactose by an engineered Saccharomyces cerevisiae strain. Appl Environ Microbiol. 77: 5822–5825. ArticlePubMedPMCLink
- Hahn-Hägerdal B, Karhumaa K, Fonseca C, Spencer-Martins I, Gorwa-Grauslund MF. 2007. Towards industrial pentose-fermenting yeast strains. Appl Microbiol Biotechnol. 74: 937–953. ArticlePubMedPDF
- Hamley-Bennett C, Lye G, Leak D. 2016. Selective fractionation of sugar beet pulp for release of fermentation and chemical feedstocks; optimisation of thermo-chemical pre-treatment. Bioresour Technol. 209: 259–264. ArticlePubMed
- Havukainen S, Pujol-Gimenez J, Valkonen M, Hediger MA, Landowski CP. 2021. Functional characterization of a highly specific L-arabinose transporter from Trichoderma reesei. Microb Cell Fact. 20: 177.ArticlePubMedPMCPDF
- Huisjes EH, de Hulster E, van Dam JC, Pronk JT, van Maris AJA. 2012a. Galacturonic acid inhibits the growth of Saccharomyces cerevisiae on galactose, xylose, and arabinose. Appl Environ Microbiol. 78: 5052–5059. ArticlePubMedPMCLink
- Huisjes EH, Luttik MA, Almering MJ, Bisschops MM, Dang DH, et al. 2012b. Toward pectin fermentation by Saccharomyces cerevisiae: expression of the first two steps of a bacterial pathway for D-galacturonate metabolism. J Biotechnol. 162: 303–310. Article
- Inokuma K, Hasunuma T, Kondo A. 2014. Efficient yeast cell-surface display of exo- and endo-cellulase using the SED1 anchoring region and its original promoter. Biotechnol Biofuels. 7: 8.ArticlePubMedPMCPDF
- Jansen MLA, Bracher JM, Papapetridis I, Verhoeven MD, de Bruijn H, et al. 2017. Saccharomyces cerevisiae strains for second-generation ethanol production: from academic exploration to industrial implementation. FEMS Yeast Res. 17: fox044.ArticlePubMedPMC
- Jansen LM, Hendriks VCA, Bentlage H, Ranoux A, Raaijmakers HWC, et al. 2024. The industrial application potential of sugar beet pulp derived monosaccharides D-galacturonic acid and L-arabinose. Chembiochem. 25: e202400521. ArticlePubMed
- Jeong D, Oh EJ, Ko JK, Nam JO, Park HS, et al. 2020. Metabolic engineering considerations for the heterologous expression of xylose-catabolic pathways in Saccharomyces cerevisiae. PLoS One. 15: e0236294. ArticlePubMedPMC
- Jeppsson M, Bengtsson O, Franke K, Lee H, Hahn-Hägerdal B, et al. 2006. The expression of a Pichia stipitis xylose reductase mutant with higher K(M) for NADPH increases ethanol production from xylose in recombinant Saccharomyces cerevisiae. Biotechnol Bioeng. 93: 665–673. ArticlePubMed
- John I, Yaragarla P, Muthaiah P, Ponnusamy K, Appusamy A. 2017. Statistical optimization of acid catalyzed steam pretreatment of citrus peel waste for bioethanol production. Resour-Efficient Technol. 3: 429–433. Article
- Karhumaa K, Garcia Sanchez R, Hahn-Hägerdal B, Gorwa-Grauslund MF. 2007. Comparison of the xylose reductase-xylitol dehydrogenase and the xylose isomerase pathways for xylose fermentation by recombinant Saccharomyces cerevisiae. Microb Cell Fact. 6: 5.ArticlePubMedPMCPDF
- Karhumaa K, Hahn-Hägerdal B, Gorwa-Grauslund MF. 2005. Investigation of limiting metabolic steps in the utilization of xylose by recombinant Saccharomyces cerevisiae using metabolic engineering. Yeast. 22: 359–368. ArticlePubMed
- Kim SR, Ha SJ, Wei N, Oh EJ, Jin YS. 2012. Simultaneous co-fermentation of mixed sugars: a promising strategy for producing cellulosic ethanol. Trends Biotechnol. 30: 274–282. ArticlePubMed
- Kim H, Oh EJ, Lane ST, Lee WH, Cate JH, et al. 2018. Enhanced cellobiose fermentation by engineered Saccharomyces cerevisiae expressing a mutant cellodextrin facilitator and cellobiose phosphorylase. J Biotechnol. 275: 53–59. ArticlePubMed
- Kim SR, Xu H, Lesmana A, Kuzmanovic U, Au M, et al. 2015. Deletion of PHO13, encoding haloacid dehalogenase type IIA phosphatase, results in upregulation of the pentose phosphate pathway in Saccharomyces cerevisiae. Appl Environ Microbiol. 81: 1601–1609. ArticlePubMedPMCLink
- Kötter P, Ciriacy M. 1993. Xylose fermentation by Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 38: 776–783. ArticlePDF
- Krahulec S, Klimacek M, Nidetzky B. 2009. Engineering of a matched pair of xylose reductase and xylitol dehydrogenase for xylose fermentation by Saccharomyces cerevisiae. Biotechnol J. 4: 684–694. ArticlePubMed
- Kuhad RC, Deswal D, Sharma S, Bhattacharya A, Jain KK, et al. 2016. Revisiting cellulase production and redefining current strategies based on major challenges. Renew Sust Energy Rev. 55: 249–272. Article
- Kumar V, Agrawal D, Bommareddy RR, Islam MA, Jacob S, et al. 2024. Arabinose as an overlooked sugar for microbial bioproduction of chemical building blocks. Crit Rev Biotechnol. 44: 1103–1120. ArticlePubMed
- Kuorelahti S, Kalkkinen N, Penttilä M, Londesborough J, Richard P. 2005. Identification in the mold Hypocrea jecorina of the first fungal D-galacturonic acid reductase. Biochemistry. 44: 11234–11240. ArticlePubMed
- Kuyper M, Hartog MM, Toirkens MJ, Almering MJ, Winkler AA, et al. 2005. Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res. 5: 399–409. ArticlePubMed
- Kyriakou M, Patsalou M, Xiaris N, Tsevis A, Koutsokeras L, et al. 2020. Enhancing bioproduction and thermotolerance in Saccharomyces cerevisiae via cell immobilization on biochar: application in a citrus peel waste biorefinery. Renew Energy. 155: 53–64. Article
- Lagunas R, Moreno E. 1992. Inhibition of glycolysis by 2-deoxygalactose in Saccharomyces cerevisiae. Yeast. 8: 107–115. ArticlePubMed
- Lane S, Xu HQ, Oh EJ, Kim H, Lesmana A, et al. 2018. Glucose repression can be alleviated by reducing glucose phosphorylation rate in Saccharomyces cerevisiae. Sci Rep. 8: 2613.ArticlePubMedPMCPDF
- Lee SH, Kodaki T, Park YC, Seo JH. 2012. Effects of NADH-preferring xylose reductase expression on ethanol production from xylose in xylose-metabolizing recombinant Saccharomyces cerevisiae. J Biotechnol. 158: 184–191. ArticlePubMed
- Lee W-H, Nan H, Kim HJ, Jin Y-S. 2013. Simultaneous saccharification and fermentation by engineered Saccharomyces cerevisiae without supplementing extracellular β-glucosidase. J Biotechnol. 167: 316–322. ArticlePubMed
- Lee SB, Tremaine M, Place M, Liu L, Pier A, et al. 2021. Crabtree/Warburg-like aerobic xylose fermentation by engineered Saccharomyces cerevisiae. Metab Eng. 68: 119–130. ArticlePubMed
- Li WJ, Fan ZG, Wu YY, Jiang ZG, Shi RC. 2019. Eco-friendly extraction and physicochemical properties of pectin from jackfruit peel waste with subcritical water. J Sci Food Agric. 99: 5283–5292. ArticlePubMedLink
- Li J, Liu G, Chen M, Li Z, Qin Y, et al. 2013. Cellodextrin transporters play important roles in cellulase induction in the cellulolytic fungus Penicillium oxalicum . Appl Microbiol Biotechnol. 97: 10479–10488. ArticlePubMedPDF
- Li J, Si H, Du H, Guo H, Dai H, et al. 2021. Comparison of gut microbiota structure and Actinobacteria abundances in healthy young adults and elderly subjects: a pilot study. BMC Microbiol. 21: 13.ArticlePubMedPMCPDF
- Li J, Xu J, Cai P, Wang B, Ma Y, et al. 2015. Functional analysis of two L-arabinose transporters from filamentous fungi reveals promising characteristics for improved pentose utilization in Saccharomyces cerevisiae. Appl Environ Microbiol. 81: 4062–4070. ArticlePubMedPMCLink
- Lian J, Li Y, HamediRad M, Zhao H. 2014. Directed evolution of a cellodextrin transporter for improved biofuel production under anaerobic conditions in Saccharomyces cerevisiae. Biotechnol Bioeng. 111: 1521–1531. ArticlePubMed
- Liu Z, Inokuma K, Ho SH, den Haan R, van Zyl WH, et al. 2017b. Improvement of ethanol production from crystalline cellulose via optimizing cellulase ratios in cellulolytic Saccharomyces cerevisiae. Biotechnol Bioeng. 114: 1201–1207. ArticleLink
- Liu G, Li B, Li C, Yuan Y. 2017a. Enhancement of simultaneous xylose and glucose utilization by regulating ZWF1 and PGI1 in Saccharomyces cerevisiae. Trans Tianjin Univ. 23: 201–210. ArticlePDF
- Lynd LR, Laser MS, Bransby D, Dale BE, Davison B, et al. 2008. How biotech can transform biofuels. Nat Biotechnol. 26: 169–172. ArticlePubMedPDF
- Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 66: 506–577. ArticlePubMedPMCLink
- Martens-Uzunova ES, Schaap PJ. 2008. An evolutionary conserved D-galacturonic acid metabolic pathway operates across filamentous fungi capable of pectin degradation. Fungal Genet Biol. 45: 1449–1457. ArticlePubMed
- Matano Y, Hasunuma T, Kondo A. 2012. Display of cellulases on the cell surface of Saccharomyces cerevisiae for high-yield ethanol production from high-solid lignocellulosic biomass. Bioresour Technol. 108: 128–133. ArticlePubMed
- Matsushika A, Goshima T, Fujii T, Inoue H, Sawayama S, et al. 2012. Characterization of non-oxidative transaldolase and transketolase enzymes in the pentose phosphate pathway with regard to xylose utilization by recombinant Saccharomyces cerevisiae. Enzyme Microb Technol. 51: 16–25. ArticlePubMed
- McDonald JE, Rooks DJ, McCarthy AJ. 2012. Methods for the isolation of cellulose-degrading microorganisms. Methods Enzymol. 510: 349–374. ArticlePubMed
- Mendez DA, Fabra MJ, Gomez-Mascaraque L, Lopez-Rubio A, Martinez-Abad A. 2021. Modelling the extraction of pectin towards the valorization of watermelon rind waste. Foods. 10: 738.ArticlePubMedPMC
- Metz B, Mojzita D, Herold S, Kubicek CP, Richard P, et al. 2013. A novel L-xylulose reductase essential for L-arabinose catabolism in Trichoderma reesei. Biochemistry. 52: 2453–2460. ArticlePubMedPMC
- Mohnen D. 2008. Pectin structure and biosynthesis. Curr Opin Plant Biol. 11: 266–277. ArticlePubMed
- Murray DB, Haynes K, Tomita M. 2011. Redox regulation in respiring Saccharomyces cerevisiae. Biochim Biophys Acta. 1810: 945–958. ArticlePubMed
- Myers KS, Riley NM, MacGilvray ME, Sato TK, McGee M, et al. 2019. Rewired cellular signaling coordinates sugar and hypoxic responses for anaerobic xylose fermentation in yeast. PLoS Genet. 15: e1008037. ArticlePubMedPMC
- Nogueira KMV, Mendes V, Kamath KS, Cheruku A, Oshiquiri LH, et al. 2024. Proteome profiling of enriched membrane-associated proteins unraveled a novel sophorose and cello-oligosaccharide transporter in Trichoderma reesei. Microb Cell Fact. 23: 22.ArticlePubMedPMCPDF
- O'Connor J, Hoang SA, Bradney L, Dutta S, Xiong X, et al. 2021. A review on the valorization of food waste as a nutrient source and soil amendment. Environ Pollut. 272: 115985.ArticlePubMed
- Oehling V, Klaassen P, Frick O, Dusny C, Schmid A. 2018. L-Arabinose triggers its own uptake via induction of the arabinose-specific Gal2p transporter in an industrial Saccharomyces cerevisiae strain. Biotechnol Biofuels. 11: 231.ArticlePubMedPMCPDF
- Oh EJ, Ha SJ, Kim SR, Lee WH, Galazka JM, et al. 2013. Enhanced xylitol production through simultaneous co-utilization of cellobiose and xylose by engineered Saccharomyces cerevisiae. Metab Eng. 15: 226–234. ArticlePubMed
- Oh EJ, Kwak S, Kim H, Jin YS. 2017. Transporter engineering for cellobiose fermentation under lower pH conditions by engineered Saccharomyces cerevisiae. Bioresour Technol. 245: 1469–1475. ArticlePubMed
- Oh EJ, Skerker JM, Kim SR, Wei N, Turner TL, et al. 2016. Gene amplification on demand accelerates cellobiose utilization in engineered Saccharomyces cerevisiae. Appl Environ Microbiol. 82: 3631–3639. ArticlePubMedPMCLink
- Orikasa Y, Mikumo D, Ohwada T. 2018. A 2-Deoxyglucose-resistant mutant of Saccharomyces cerevisiae shows enhanced maltose fermentative ability by the activation of MAL genes. Foods. 7: 52.ArticlePubMedPMC
- Osiro KO, Borgstrom C, Brink DP, Fjolnisdottir BL, Gorwa-Grauslund MF. 2019. Exploring the xylose paradox in Saccharomyces cerevisiae through in vivo sugar signalomics of targeted deletants. Microb Cell Fact. 18: 88.ArticlePubMedPMCPDF
- Parisutham V, Chandran SP, Mukhopadhyay A, Lee SK, Keasling JD. 2017. Intracellular cellobiose metabolism and its applications in lignocellulose-based biorefineries. Bioresour Technol. 239: 496–506. ArticlePubMed
- Pathania S, Sharma N, Handa S. 2018. Utilization of horticultural waste (Apple Pomace) for multiple carbohydrase production from Rhizopus delemar F2 under solid-state fermentation. J Genet Eng Biotechnol. 16: 181–189. ArticlePubMedPMC
- Pocan P, Bahcegul E, Oztop MH, Hamamci H. 2018. Enzymatic hydrolysis of fruit peels and other lignocellulosic biomass as a source of sugar. Waste Biomass Valorization. 9: 929–937. ArticlePDF
- Pourbafrani M, Forgacs G, Horvath IS, Niklasson C, Taherzadeh MJ. 2010. Production of biofuels, limonene, and pectin from citrus wastes. Bioresour Technol. 101: 4246–4250. ArticlePubMed
- Protzko RJ, Hach CA, Coradetti ST, Hackhofer MA, Magosch S, et al. 2019. Genome-wide and enzymatic analysis reveals efficient D-galacturonic acid metabolism in the basidiomycete yeast Rhodosporidium toruloides. mSystems. 4: e00389-19.ArticlePubMedPMC
- Protzko RJ, Latimer LN, Martinho Z, de Reus E, Seibert T, et al. 2018. Engineering Saccharomyces cerevisiae for co-utilization of D-galacturonic acid and D-glucose from citrus peel waste. Nat Commun. 9: 5059.ArticlePubMedPMCPDF
- Qi X, Zha J, Liu GG, Zhang W, Li BZ, et al. 2015. Heterologous xylose isomerase pathway and evolutionary engineering improve xylose utilization in Saccharomyces cerevisiae. Front Microbiol. 6: 1165.ArticlePubMedPMC
- Rabha J, Devi SP, Das S, Roy N, Jha DK. 2023. Microbial conversion of biomass to value-added chemicals. In Kuddus M, Ramteke P. (eds.), Value-Addition in Agri-food Industry Waste Through Enzyme Technology, pp. 37-64. Academic Press.Article
- Reddy LV, Obulam VSR, Wee YJ. 2011. Production of ethanol from mango (Mangifera indica L.) peel by Saccharomyces cerevisiae CFTRI101. Afr J Biotechnol. 10: 4183–4189.Link
- Richard P, Hilditch S. 2009. D-Galacturonic acid catabolism in microorganisms and its biotechnological relevance. Appl Microbiol Biotechnol. 82: 597–604. ArticlePubMedPDF
- Richard P, Verho R, Putkonen M, Londesborough J, Penttilä M. 2003. Production of ethanol from L-arabinose by Saccharomyces cerevisiae containing a fungal L-arabinose pathway. FEMS Yeast Res. 3: 185–189. ArticlePubMed
- Rincón AM, Codón AC, Castrejón F, Benítez T. 2001. Improved properties of baker’s yeast mutants resistant to 2-deoxy-D-glucose. Appl Environ Microbiol. 67: 4279–4285. ArticlePubMedPMCLink
- Rizzi M, Klein C, Schulze C, Bui-Thanh NA, Dellweg H. 1989. Xylose fermentation by yeasts. 5. Use of ATP balances for modeling oxygen-limited growth and fermentation of yeast Pichia stipitis with xylose as carbon source. Biotechnol Bioeng. 34: 509–514. ArticlePubMed
- Roca C, Haack MB, Olsson L. 2004. Engineering of carbon catabolite repression in recombinant xylose-fermenting Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 63: 578–583. ArticlePubMedPDF
- Runquist D, Hahn-Hagerdal B, Bettiga M. 2010. Increased ethanol productivity in xylose-utilizing Saccharomyces cerevisiae via a randomly mutagenized xylose reductase. Appl Environ Microbiol. 76: 7796–7802. ArticlePubMedPMCLink
- Sato TK, Tremaine M, Parreiras LS, Hebert AS, Myers KS, et al. 2016. Directed evolution reveals unexpected epistatic interactions that alter metabolic regulation and enable anaerobic xylose use by Saccharomyces cerevisiae. PLoS Genet. 12: e1006372. ArticlePubMedPMC
- Soncini SR, Chandrashekarappa DG, Augustine DA, Callahan KP, O'Donnell AF, et al. 2020. Spontaneous mutations that confer resistance to 2-deoxyglucose act through Hxk2 and Snf1 pathways to regulate gene expression and HXT endocytosis. PLoS Genet. 16: e1008484. ArticlePubMedPMC
- Souto-Maior AM, Runquist D, Hahn-Hagerdal B. 2009. Crabtree-negative characteristics of recombinant xylose-utilizing Saccharomyces cerevisiae. J Biotechnol. 143: 119–123. ArticlePubMed
- Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, et al. 2015. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 90: 927–963. ArticlePubMedPMCLink
- Subtil T, Boles E. 2011. Improving L-arabinose utilization of pentose-fermenting Saccharomyces cerevisiae cells by heterologous expression of L-arabinose transporting sugar transporters. Biotechnol Biofuels. 4: 38.ArticlePubMedPMC
- Taghizadeh-Alisaraei A, Hosseini SH, Ghobadian B, Motevali A. 2017. Biofuel production from citrus wastes: A feasibility study in Iran. Renew. Sustain. Energy Rev. 69: 1100–1112. Article
- Thevelein JM, de Winde JH. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 33: 904–918. ArticlePubMed
- Tran PHN, Ko JK, Gong GT, Um Y, Lee SM. 2020. Improved simultaneous co-fermentation of glucose and xylose by Saccharomyces cerevisiae for efficient lignocellulosic biorefinery. Biotechnol Biofuels. 13: 12.ArticlePubMedPMC
- Trumbly RJ. 1992. Glucose repression in the yeast Saccharomyces cerevisiae. Mol Microbiol. 6: 15–21. ArticlePubMed
- Tsai SL, DaSilva NA, Chen W. 2013. Functional display of complex cellulosomes on the yeast surface via adaptive assembly. ACS Synth Biol. 2: 14–21. ArticlePubMed
- Turner TL, Zhang GC, Oh EJ, Subramaniam V, Adiputra A, et al. 2016. Lactic acid production from cellobiose and xylose by engineered Saccharomyces cerevisiae. Biotechnol Bioeng. 113: 1075–1083. ArticlePubMed
- UNEP. 2024. Food Waste Index Report 2024. Think Eat Save: Tracking Progress to Halve Global Food Waste.Link
- Valk LC, Luttik MAH, de Ram C, Pabst M, van den Broek M, et al. 2019. A novel D-galacturonate fermentation pathway in Lactobacillus suebicus links initial reactions of the galacturonate-isomerase route with the phosphoketolase pathway. Front Microbiol. 10: 3027.ArticlePubMedPMC
- Vera AM, Galera-Prat A, Wojciechowski M, Różycki B, Laurents DV, et al. 2021. Cohesin-dockerin code in cellulosomal dual binding modes and its allosteric regulation by proline isomerization. Structure. 29: 587–597. ArticlePubMed
- Verduyn C, Van Kleef R, Frank J, Schreuder H, Van Dijken JP, et al. 1985. Properties of the NAD(P)H-dependent xylose reductase from the xylose-fermenting yeast Pichia stipitis . Biochem J. 226: 669–677. ArticlePubMedPMCPDF
- Verho R, Putkonen M, Londesborough J, Penttila M, Richard P. 2004. A novel NADH-linked L-xylulose reductase in the L-arabinose catabolic pathway of yeast. J Biol Chem. 279: 14746–14751. ArticlePubMed
- Verhoeven MD, de Valk SC, Daran JG, van Maris AJA, Pronk JT. 2018. Fermentation of glucose-xylose-arabinose mixtures by a synthetic consortium of single-sugar-fermenting Saccharomyces cerevisiae strains. FEMS Yeast Res. 18: foy075.Article
- Wagner J, Schafer D, von den Eichen N, Haimerl C, Harth S, et al. 2021. D-Galacturonic acid reduction by S. cerevisiae for L-galactonate production from extracted sugar beet press pulp hydrolysate. Appl Microbiol Biotechnol. 105: 5795–5807. ArticlePubMedPMCPDF
- Walfridsson M, Hallborn J, Penttila M, Keranen S, Hahn-Hagerdal B. 1995. Xylose-metabolizing Saccharomyces cerevisiae strains overexpressing the TKL1 and TAL1 genes encoding the pentose phosphate pathway enzymes transketolase and transaldolase. Appl Environ Microbiol. 61: 4184–4190. ArticlePubMedPMCLink
- Wang C, Li Y, Qiu C, Wang S, Ma J, et al. 2017a. Identification of important amino acids in Gal2p for improving L-arabinose transport and metabolism in Saccharomyces cerevisiae. Front Microbiol. 8: 1391.Article
- Wang C, Shen Y, Zhang Y, Suo F, Hou J, et al. 2013. Improvement of L-arabinose fermentation by modifying the metabolic pathway and transport in Saccharomyces cerevisiae. Biomed Res Int. 2013: 461204.ArticlePubMedPMCPDF
- Wang X, Yang J, Yang S, Jiang Y. 2019. Unraveling the genetic basis of fast L-arabinose consumption on top of recombinant xylose-fermenting Saccharomyces cerevisiae. Biotechnol Bioeng. 116: 283–293. ArticlePubMed
- Wang C, Zhao J, Qiu C, Wang S, Shen Y, et al. 2017b. Co-utilization of D-glucose, D-xylose, and L-arabinose in Saccharomyces cerevisiae by co-expressing the metabolic pathways and evolutionary engineering. Biomed Res Int. 2017: 5318232.Article
- Wen F, Sun J, Zhao H. 2010. Yeast surface display of trifunctional minicellulosomes for simultaneous saccharification and fermentation of cellulose to ethanol. Appl Environ Microbiol. 76: 1251–1260. ArticlePubMedPMCLink
- Widmer W, Zhou W, Grohmann K. 2010. Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Bioresour Technol. 101: 5242–5249. ArticlePubMed
- Wiedemann B, Boles E. 2008. Codon-optimized bacterial genes improve L-arabinose fermentation in recombinant Saccharomyces cerevisiae. Appl Environ Microbiol. 74: 2043–2050. ArticlePubMedPMCLink
- Wilkins MR, Widmer WW, Grohmann K. 2007. Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem. 42: 1614–1619. Article
- Wilson DB. 2011. Microbial diversity of cellulose hydrolysis. Curr Opin Microbiol. 14: 259–263. ArticlePubMed
- Wu M, Li H, Wei S, Wu H, Wu X, et al. 2020. Simulating extracellular glucose signals enhances xylose metabolism in recombinant Saccharomyces cerevisiae. Microorganisms. 8: 100.ArticlePubMedPMC
- Xiao C, Anderson CT. 2013. Roles of pectin in biomass yield and processing for biofuels. Front Plant Sci. 4: 67.ArticlePubMedPMC
- Xu JR, Zhao XQ, Liu CG, Bai FW. 2018. Improving xylose utilization of Saccharomyces cerevisiae by expressing the MIG1 mutant from the self-flocculating yeast SPSC01 . Protein Pept Lett. 25: 202–207. ArticlePubMed
- Yang G, Tan H, Li S, Zhang M, Che J, et al. 2020. Application of engineered yeast strain fermentation for oligogalacturonides production from pectin-rich waste biomass. Bioresour Technol. 300: 122645.ArticlePubMed
- Yang P, Wu Y, Zheng Z, Cao L, Zhu X, et al. 2018. CRISPR-Cas9 approach constructing cellulase sestc-engineered Saccharomyces cerevisiae for the production of orange peel ethanol. Front Microbiol. 9: 2436.ArticlePubMedPMC
- Yapo BM, Lerouge P, Thibault J-F, Ralet M-C. 2007. Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydr Polym. 69: 426–435. Article
- Yuan Y, Zhao H. 2013. Directed evolution of a highly efficient cellobiose-utilizing pathway in an industrial Saccharomyces cerevisiae strain. Biotechnol Bioeng. 110: 2874–2881. ArticlePubMed
- Zannini D, Dal Poggetto G, Malinconico M, Santagata G, Immirzi B. 2021. Citrus pomace biomass as a source of pectin and lignocellulose fibers: From waste to upgraded biocomposites for mulching applications. Polymers. 13: 1280.ArticlePubMedPMC
- Zha J, Li BZ, Shen MH, Hu ML, Song H, et al. 2013. Optimization of CDT-1 and XYL1 expression for balanced co-production of ethanol and xylitol from cellobiose and xylose by engineered Saccharomyces cerevisiae. PLoS One. 8: e68317. ArticlePubMedPMC
- Zha J, Shen M, Hu M, Song H, Yuan Y. 2014. Enhanced expression of genes involved in initial xylose metabolism and the oxidative pentose phosphate pathway in the improved xylose-utilizing Saccharomyces cerevisiae through evolutionary engineering. J Ind Microbiol Biotechnol. 41: 27–39. ArticlePubMedPDF
- Zhang W, Kou Y, Xu J, Cao Y, Zhao G. 2013. Two major facilitator superfamily sugar transporters from Trichoderma reesei and their roles in induction of cellulase biosynthesis. J Biol Chem. 288: 32861–32872. ArticlePubMedPMC
- Zhao L, Wu L, Li L, Zhu J, Chen X, et al. 2023. Physicochemical, structural, and rheological characteristics of pectic polysaccharides from fresh passion fruit (Passiflora edulis f. flavicarpa L.) peel. Food Hydrocoll. 136: 108301.Article
- Zhou W, Widmer W, Grohmann K. 2008. Developments in ethanol production from citrus peel waste. Proc Fla State Hortic Soc. 121: 307–310.Link
- Zhu J, Zhang K, He Y, Zhang Q, Ran Y, et al. 2024. Metabolic engineering of Saccharomyces cerevisiae for chelerythrine biosynthesis. Microb Cell Fact. 23: 183.ArticlePubMedPMCPDF
- Zieminski K, Kowalska-Wentel M. 2017. Effect of different sugar beet pulp pretreatments on biogas production efficiency. Appl Biochem Biotechnol. 181: 1211–1227. ArticlePubMedPMCPDF
- Znameroski EA, Li X, Tsai JC, Galazka JM, Glass NL, et al. 2014. Evidence for transceptor function of cellodextrin transporters in Neurospora crassa. J Biol Chem. 289: 2610–2619. ArticlePubMedPMC
References
Figure & Data
References
Citations

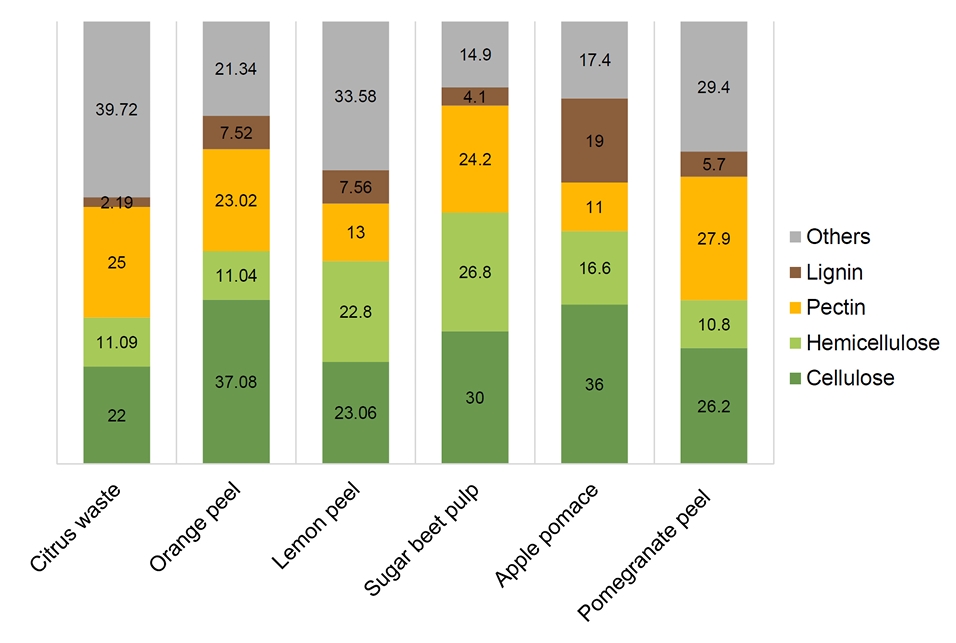
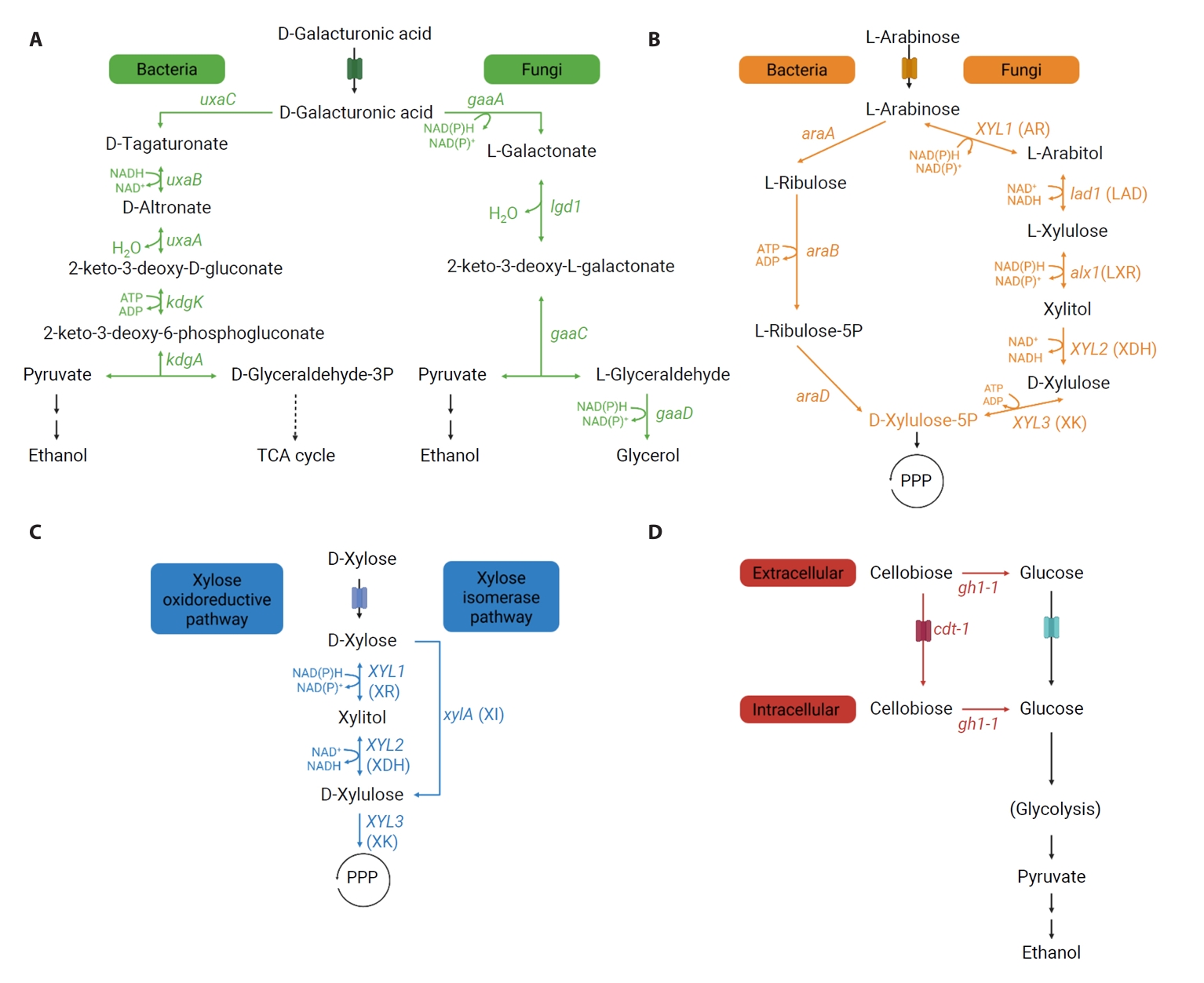
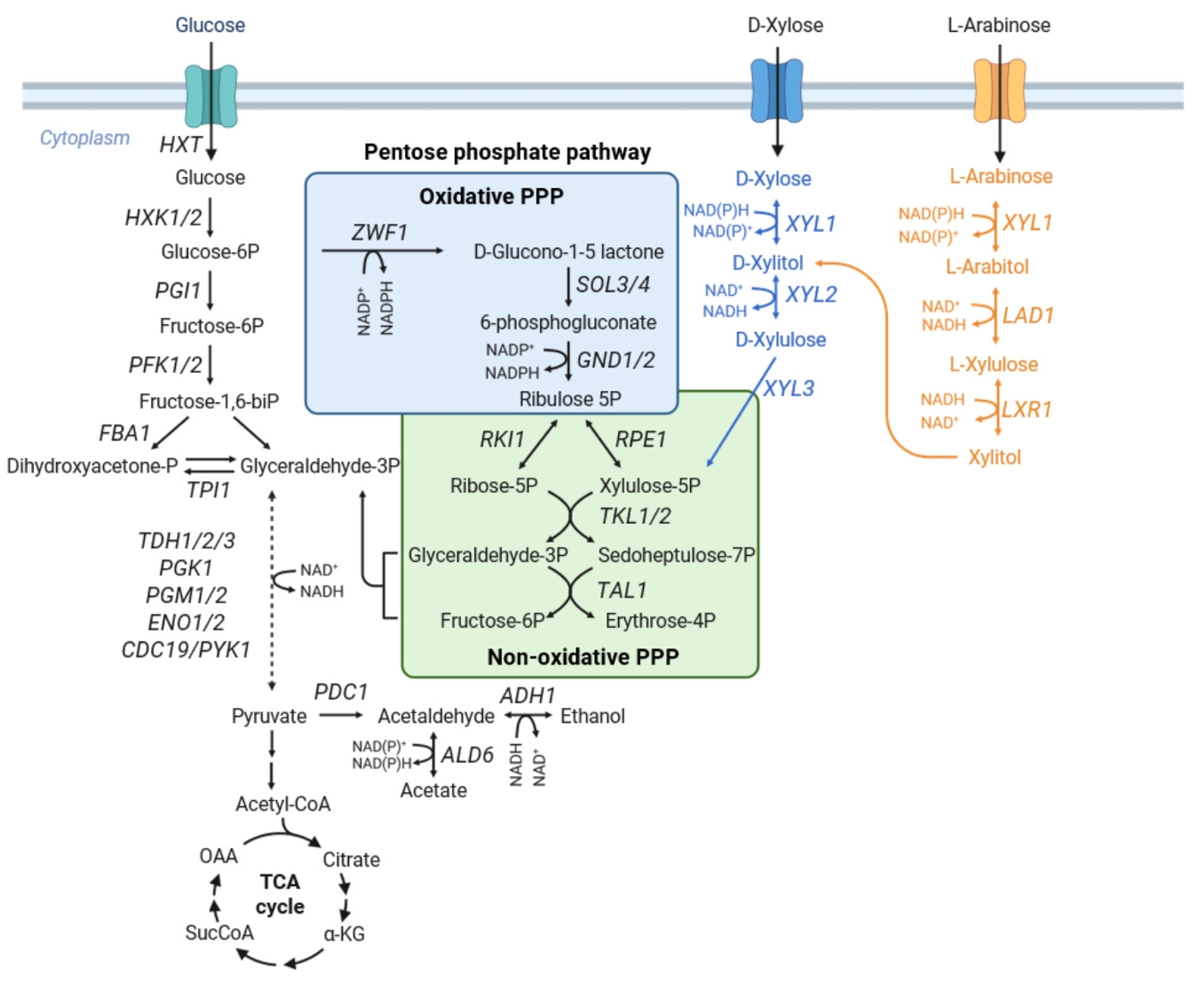
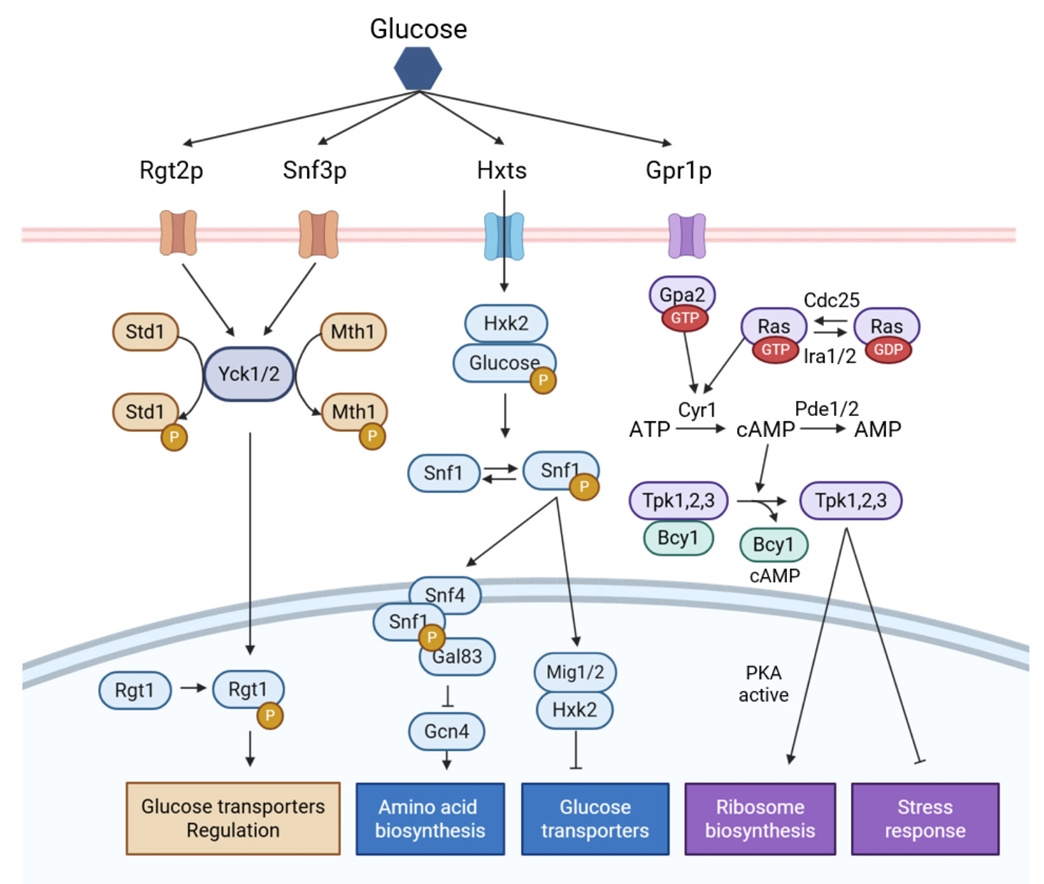
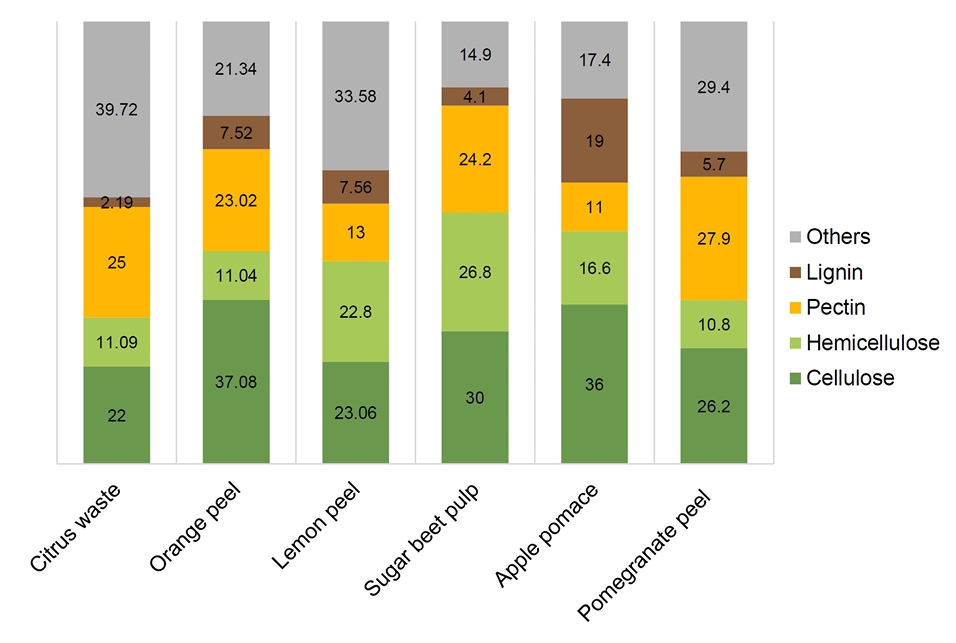
Fig. 1.
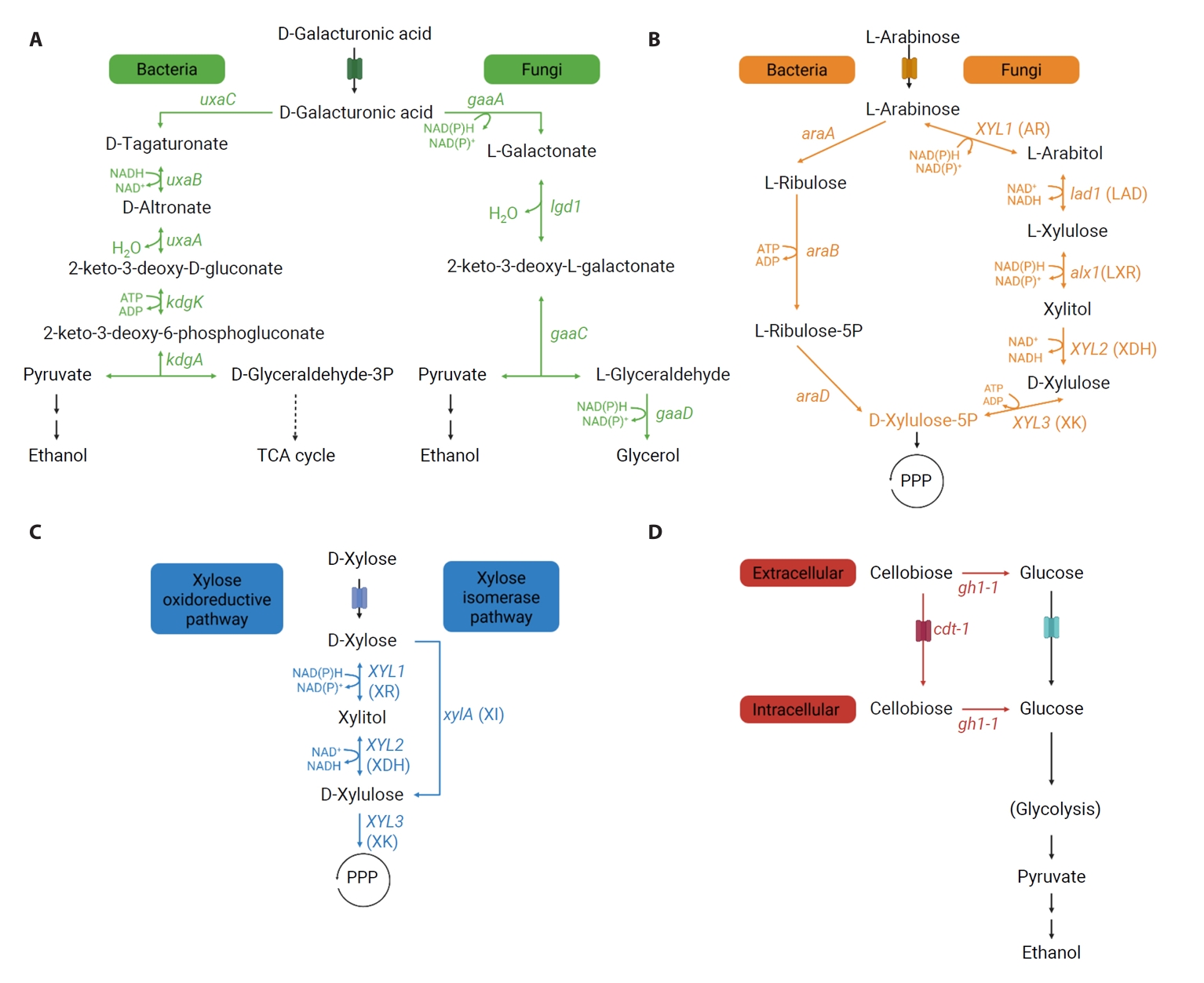
Fig. 2.
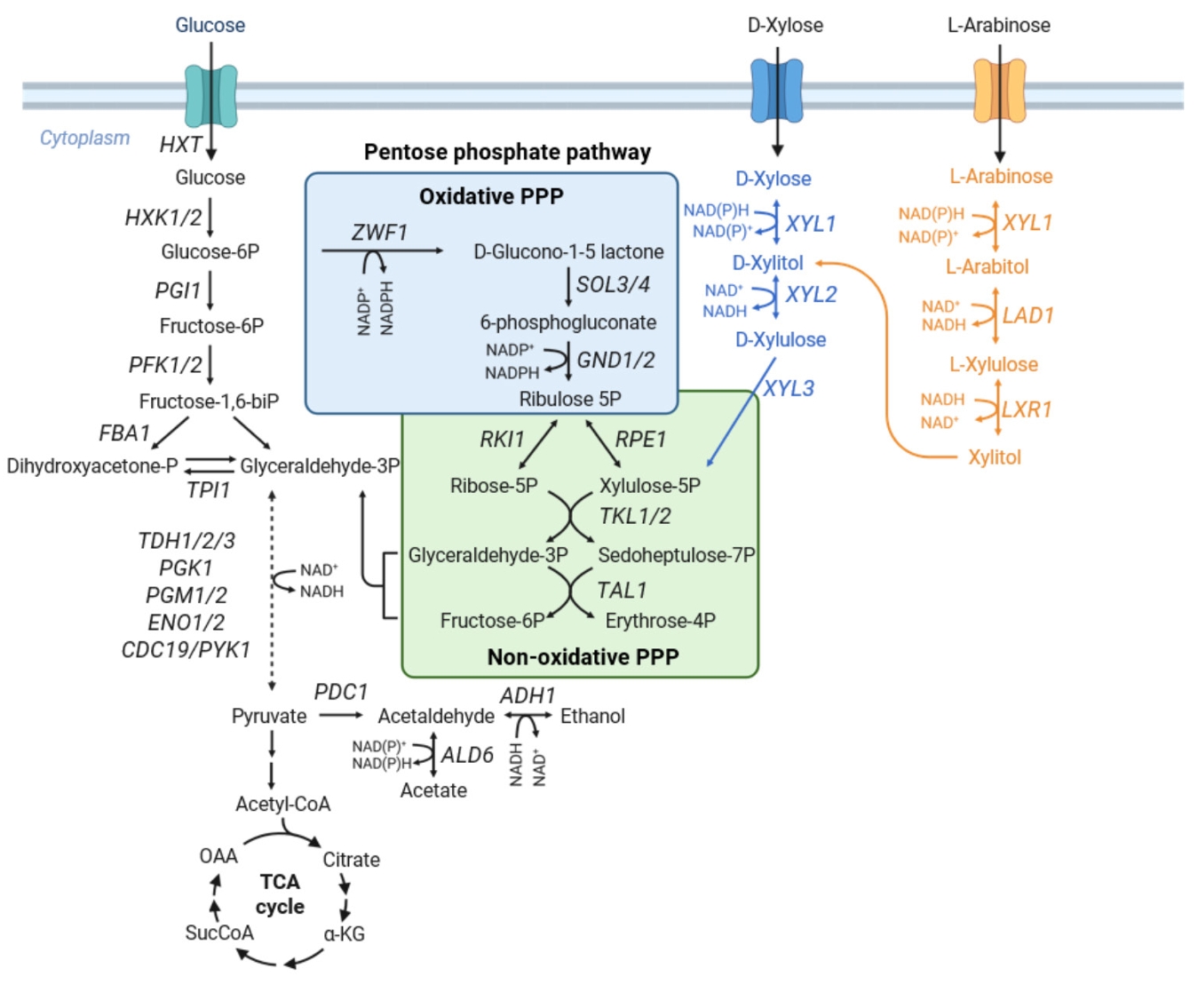
Fig. 3.
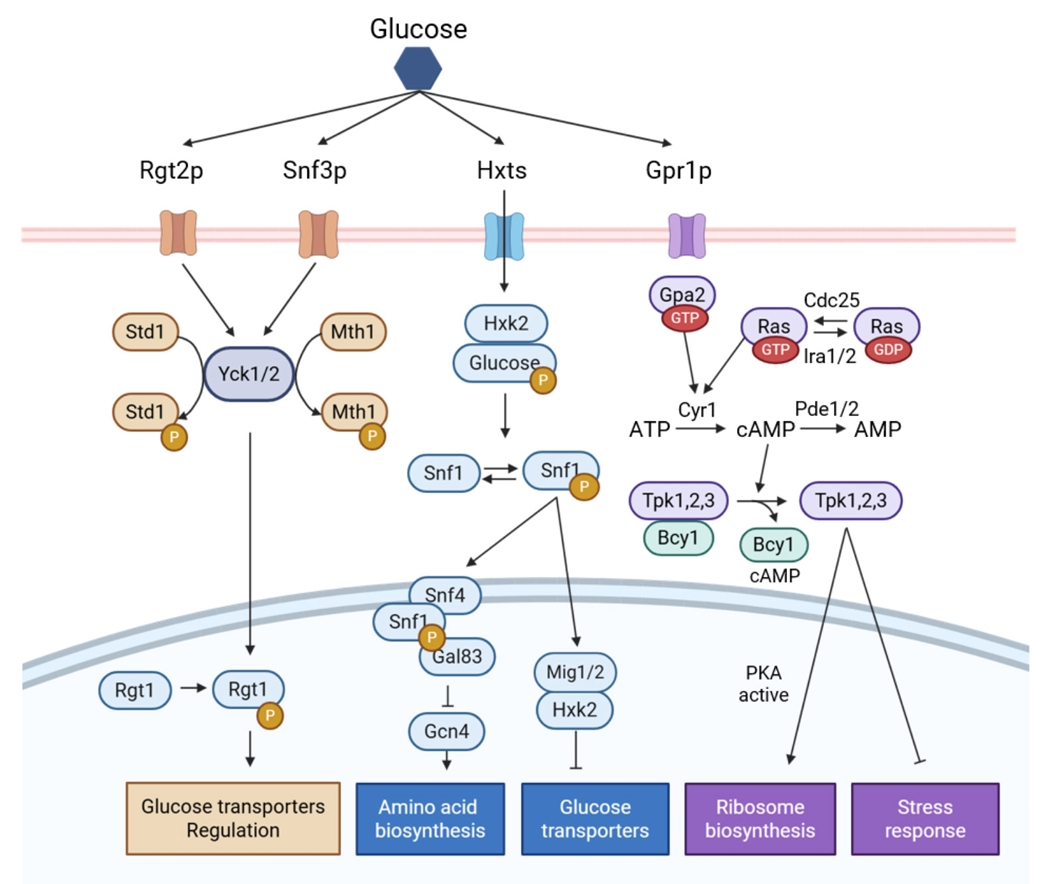
Fig. 4.
| Feedstock | Pretreatment | Hydrolysis | Glc | GalUA | Ara | Gal | Xyl | Rha | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Sugar beet pulp | Steam pretreatment at 5 bar pressure for 24 min | Enzymatic hydrolysis using cellulase 13L-C013L | 25.9 | 14.4 | 23 | 6.2 | 1.7 | 2.4 | |
| Apple pomace | Acid pretreatment, 72% sulfuric acid 16 min at 91°C | Viscozyme L and celluclast mixture for 100 h | 13.1 | 20.6 | 10.9 | 3.1 | - | - | |
| Mandarin peel | UV sterilization, freeze-dried | Polygalacturonase expressed by engineered, Pichia pastoris | 39.4 | 27.3 | 3.3 | 3.9 | 2.4 | 2.9 | |
| Orange peel | UV sterilization, freeze-dried | Polygalacturonase expressed by engineered, Pichia pastoris | 35.5 | 25.6 | 5.6 | 2.7 | 2.2 | 2.1 | |
| Jackfruit peel | Blanched, 80% (v/v) ethanol | 2 M H2SO4 110°C for 6 h | 3.5 | 57.0 | 5.2 | 23.3 | - | 9.7 | |
| Melon peel | Microwave-assisted extraction with hexane and ethanol | Trifluoroacetic acid at 120°C for 75 min | 14.4 | 40.8 | 30.1 | 8.3 | - | 1.2 | |
| Passion fruit peel | Ultrasound-microwave-assisted three-phase partitioning | Trifluoroacetic acid at 110°C for 6 h | 8.3 | 56.6 | 25.3 | 7.9 | - | 9.0 | |
| Watermelon rind | Freeze-dried, 96% (v/v) ethanol | 2 M HCl in dry methanol for 5 h at 100°C | 57.1 | 26.9 | 2.8 | 17.9 | 6.2 | 1.5 |
| Pectin-rich residue | Fermentation type | Hydrolysis | Fermentable sugars |
pH | Ethanol yield (g/g) | Productivity (g/L/h) | Time (h) | Reference |
|---|---|---|---|---|---|---|---|---|
| Orange peel citrus waste | SSF | Pectinase, cellulase, β-glucosidase | 5.93 | 6 | 0.67 | 1.50 | 24 | |
| Orange peel citrus waste | SSF | Pectinase, cellulase, β-glucosidase | - | 4.2–4.8 | 4.05% (w/v) | - | 18 | |
| Mango peel hydrolysate | SHF | Pectinase, trizyme 50 | 150 | 5 | 0.476 | 0.99 | 72 | |
| Mandarin peel | SHF | In-house from enzyme HEA and HEB | 68.5 | 4.8 | 0.43 | 3.28 | 9 | |
| Grapefruit peel | SHF | In-house from enzyme HEA and HEB | 51.8 | 4.8 | 0.42 | 2.40 | 9 | |
| Lemon peel | SHF | In-house from enzyme HEA and HEB | 46.8 | 4.8 | 0.42 | 2.18 | 9 | |
| Lime peel | SHF | In-house from enzyme HEA and HEB | 34.8 | 4.8 | 0.41 | 1.60 | 9 | |
| Orange peel | CBP | Genome integration of the cellulase gene from Ampullaria gigas Spix | 20.0 | - | 0.38 | 0.16 | 48 | |
| Orange processing waste | SSF | Pectinase, cellulase | 57.7 | 4.2 | 0.48 | 0.56 | 48 | |
| Sweet lime peel | SHF | Pectinase, cellulase | 7.10 | - | 0.092 | - | 72 | |
| Citrus peel waste | SHF | Acid hydrolysis | 101 | 4.8 | 0.63 | 3.42 | 9 | |
| Orange citrus waste | SHF | Acid hydrolysis | 29.0 | 5 | 0.43 | 1.65 | 24 |
Content (%, w/w); g/100 g dry matter Parameters: Glc, glucose; GalUA, galacturonic acid; Ara, arabinose ; Gal, galactose; Xyl, xylose; Rha, rhamnose
SSF, Simultaneous Saccharification and Fermentation; SHF, Separate Hydrolysis and Fermentation; CBP, Consolidated Bioprocessing; In-house enzyme from Sum of fermentable sugars (sucrose, glucose, fructose, and galactose)
Table 1.
Table 2.
TOP
 MSK
MSK

 Cite this Article
Cite this Article