Articles
- Page Path
- HOME > J. Microbiol > Volume 63(4); 2025 > Article
-
Full article
Antifungal effects of Metformin against Candida albicans by autophagy regulation - Xiao Zhao1,2,3, Yang Wang1,2,3, Qinqin Zhang1,2,3, Yun Huang1,2,3, Xin Wei1,2,3,*, Daming Wu1,2,3,*
-
Journal of Microbiology 2025;63(4):e2411008.
DOI: https://doi.org/10.71150/jm.2411008
Published online: April 29, 2025
1Department of Endodontics, The Affiliated Stomatological Hospital of Nanjing Medical University, Nanjing 210000, P. R. China
2Jiangsu Province Engineering Research Center of Stomatological Translational Medicine, Nanjing 210000, P. R. China
3State Key Laboratory Cultivation Base of Research, Prevention and Treatment for Oral Diseases, Nanjing 210000, P. R. China
- *Correspondence Xin Wei weixinart@163.com Daming Wu wdming@njmu.edu.cn
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
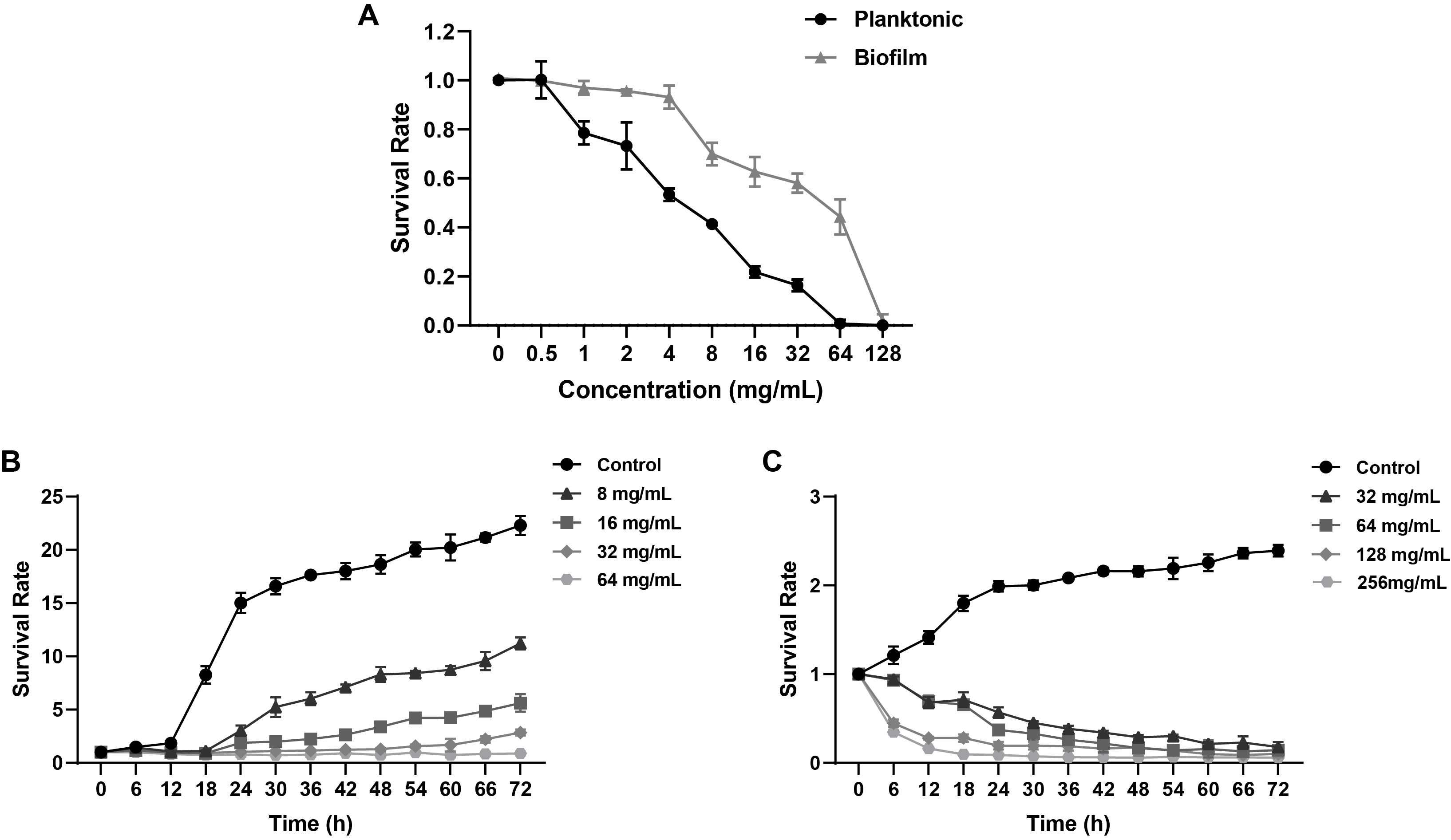
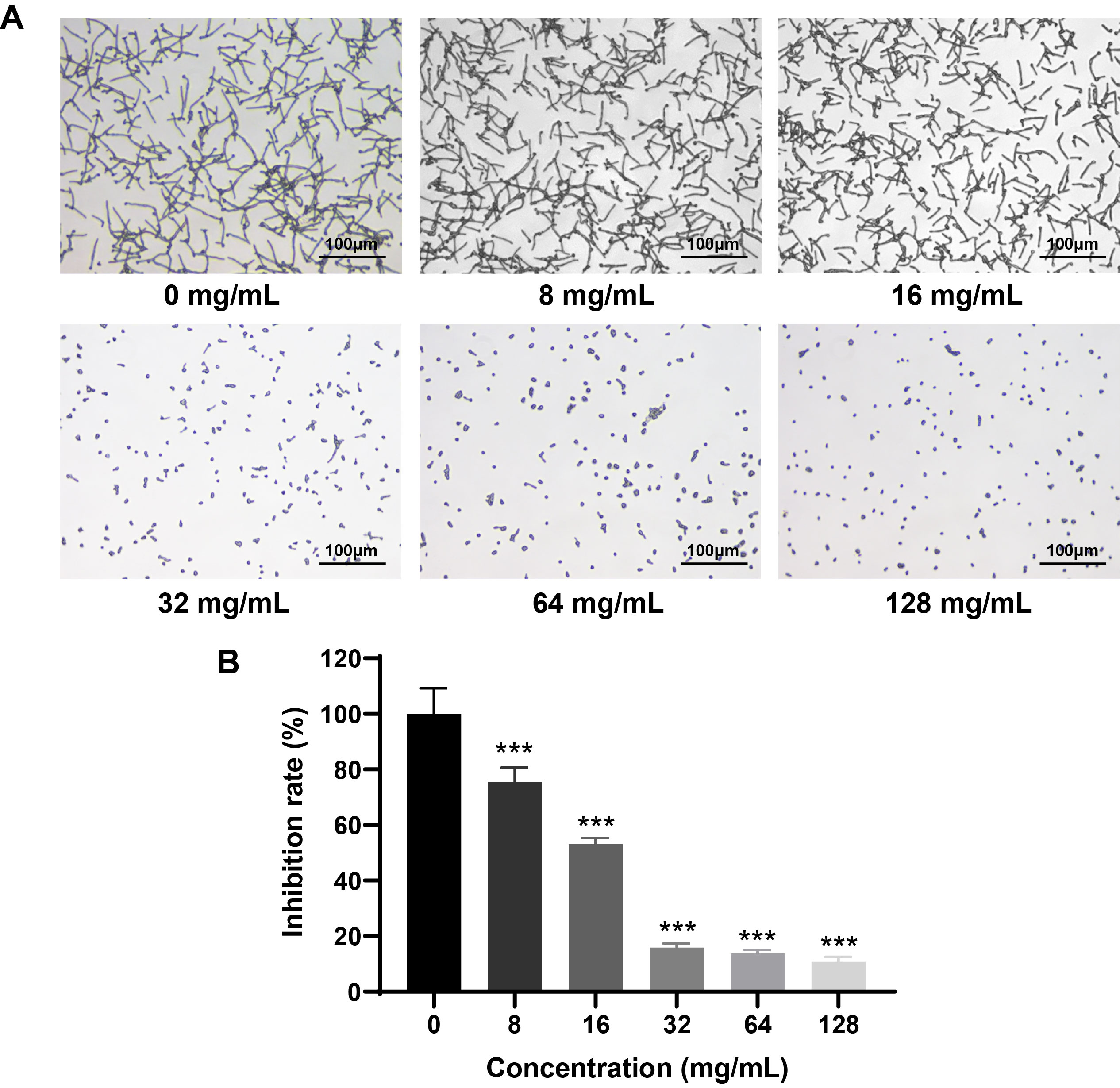
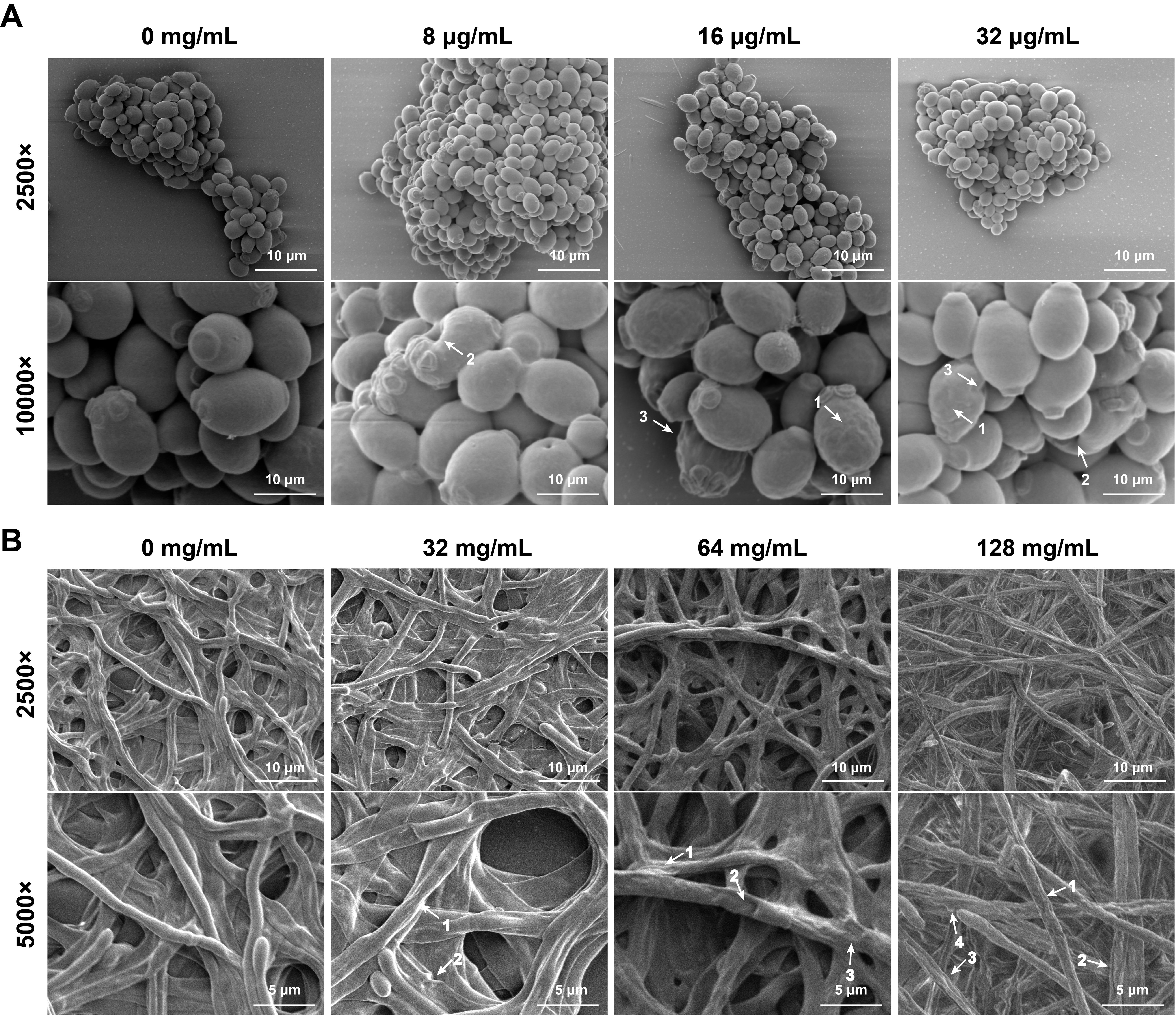
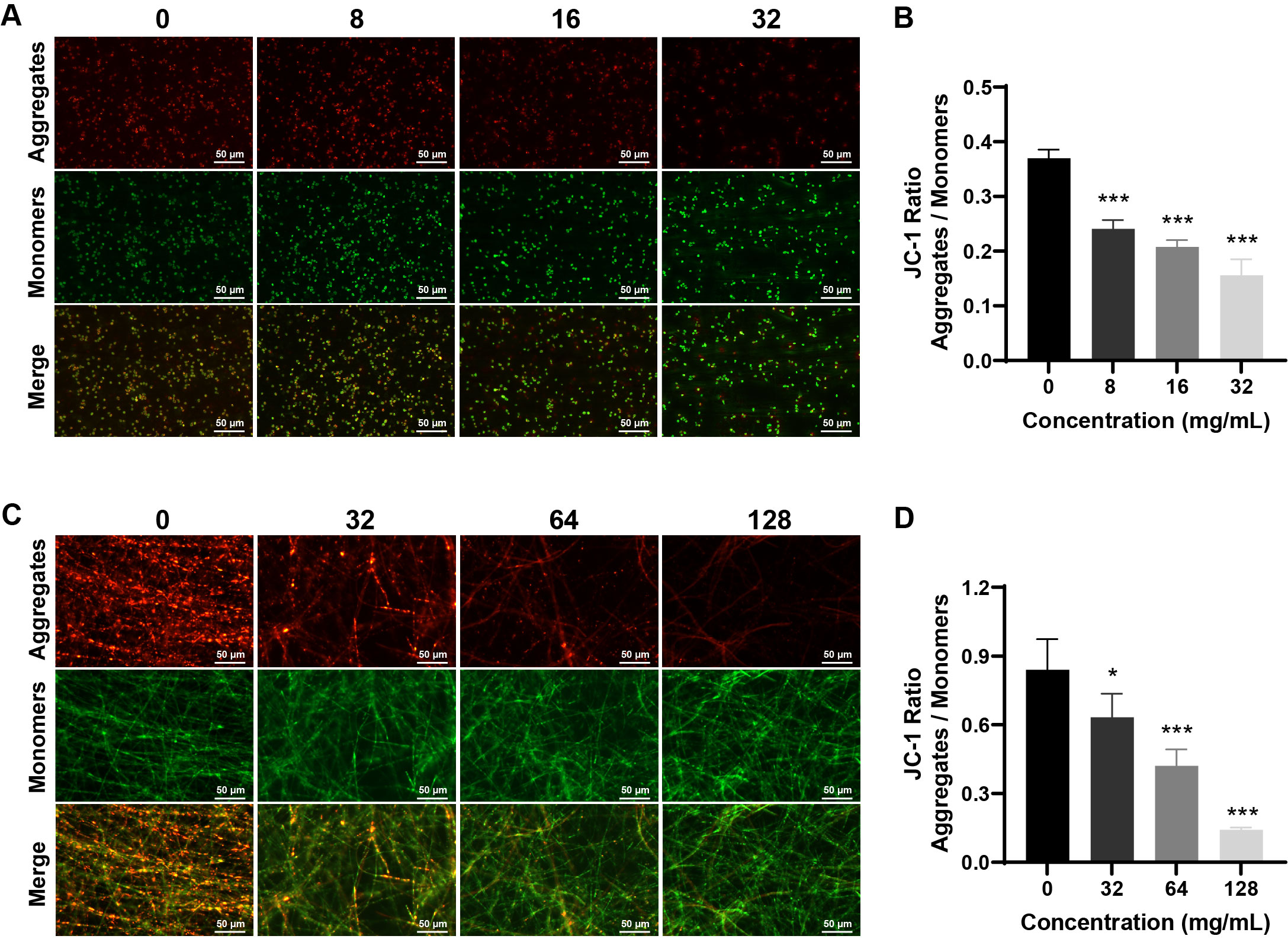
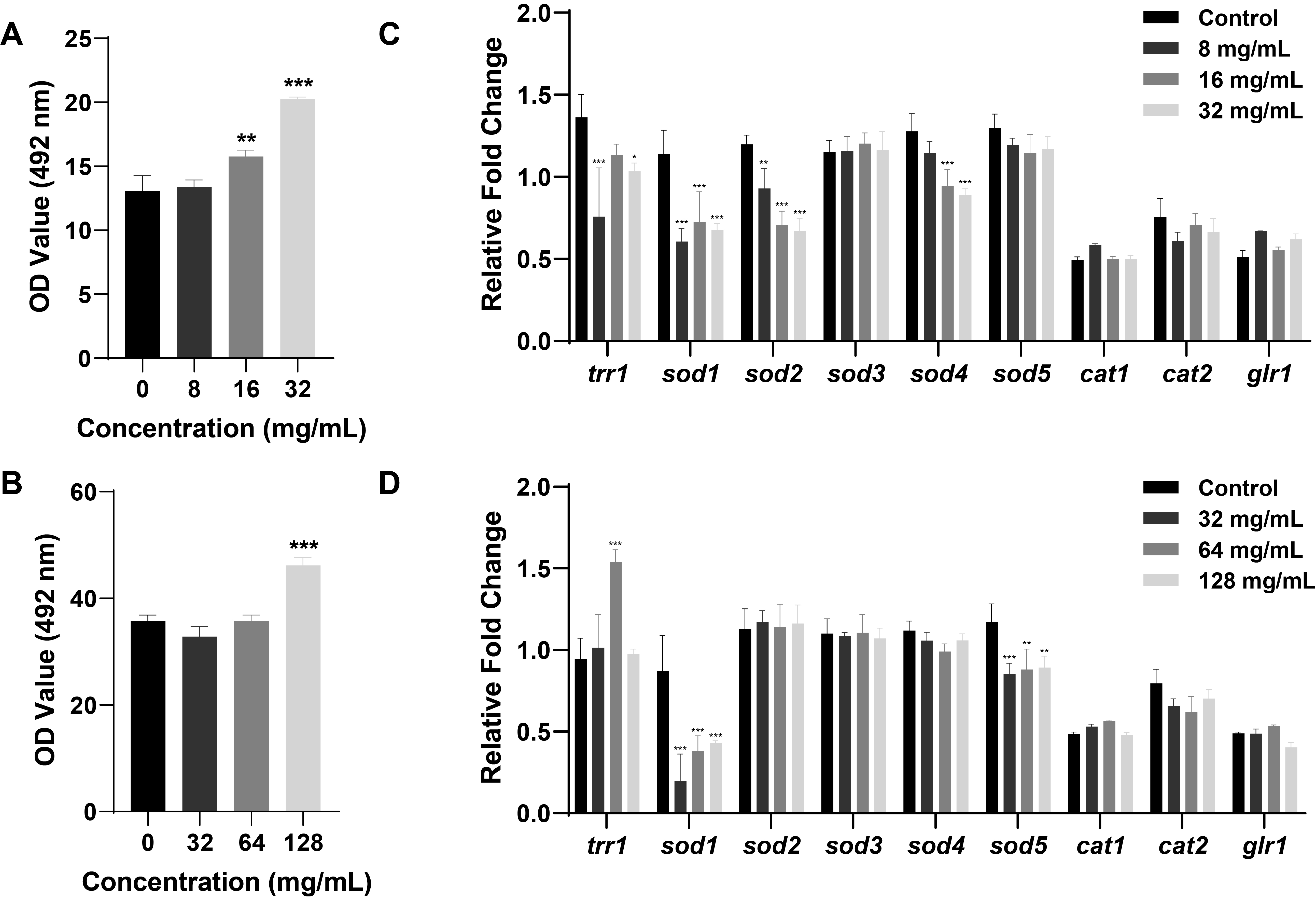
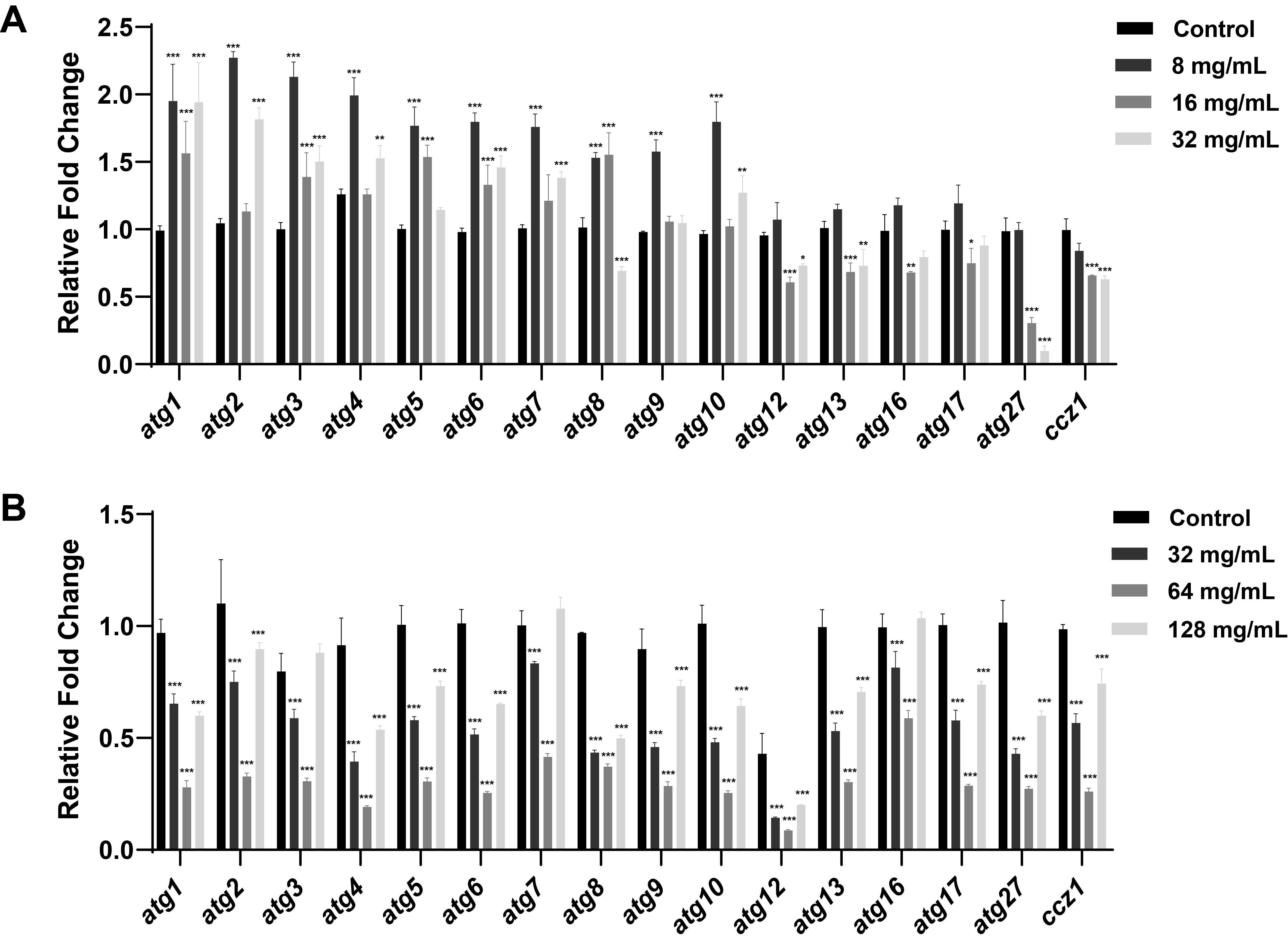
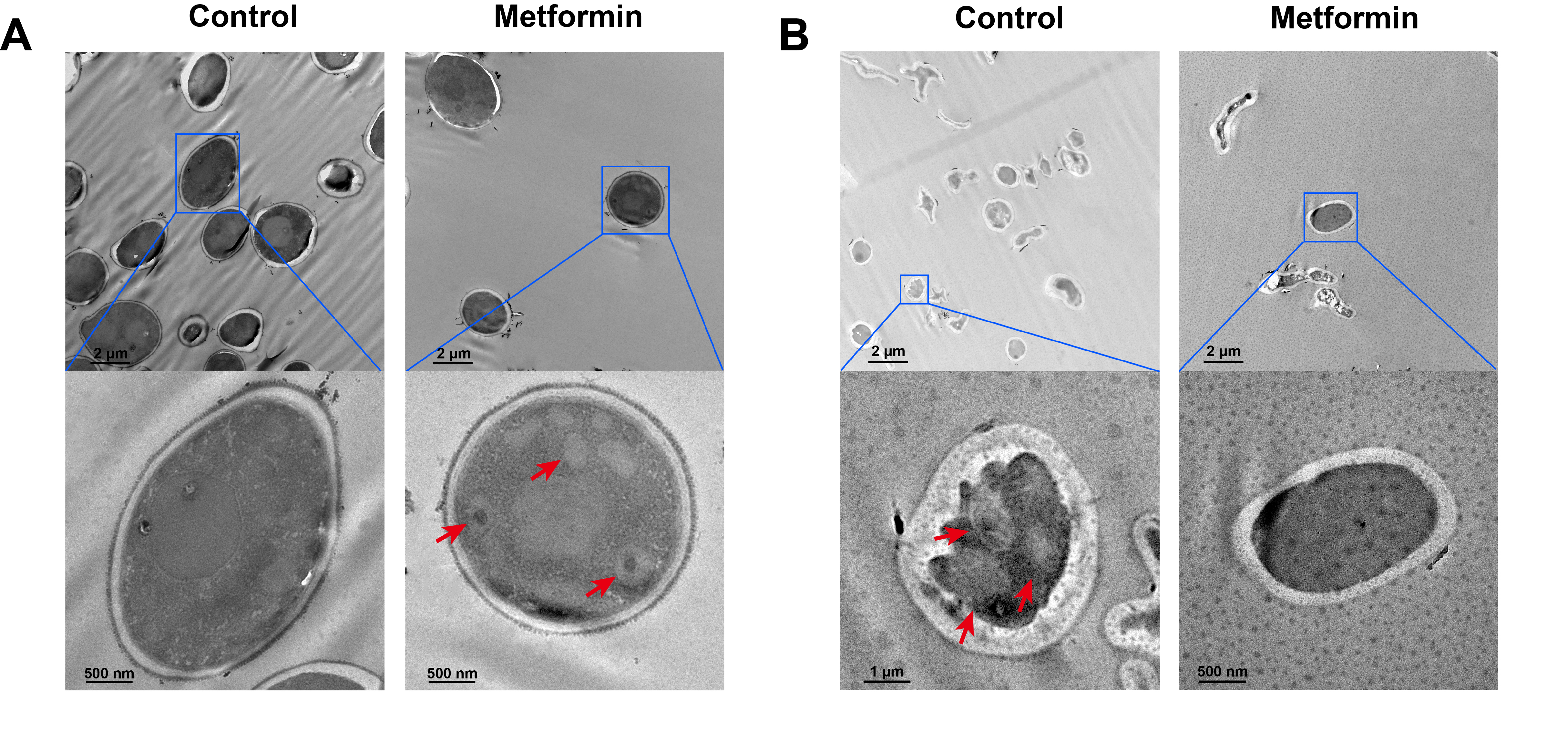
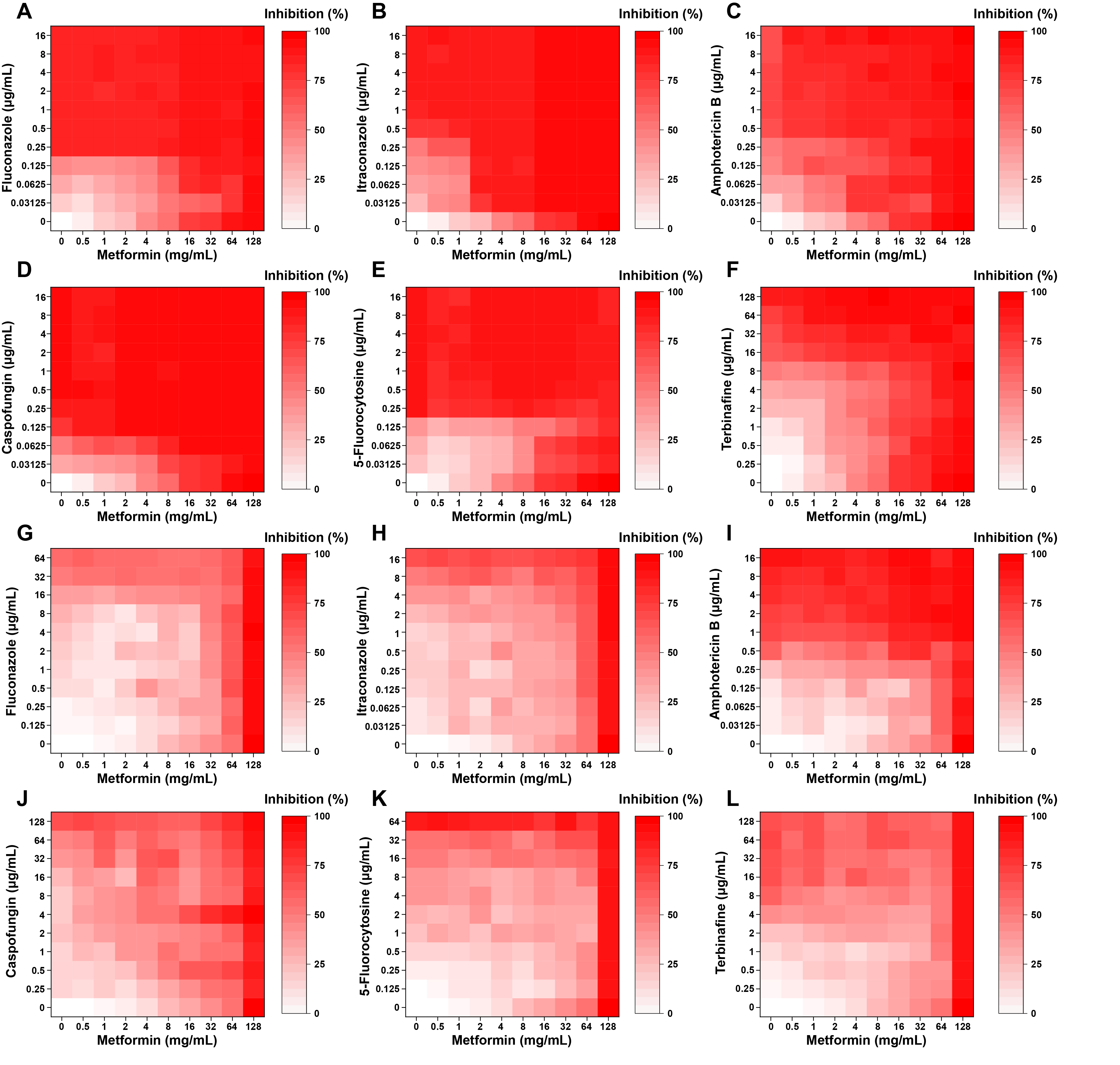
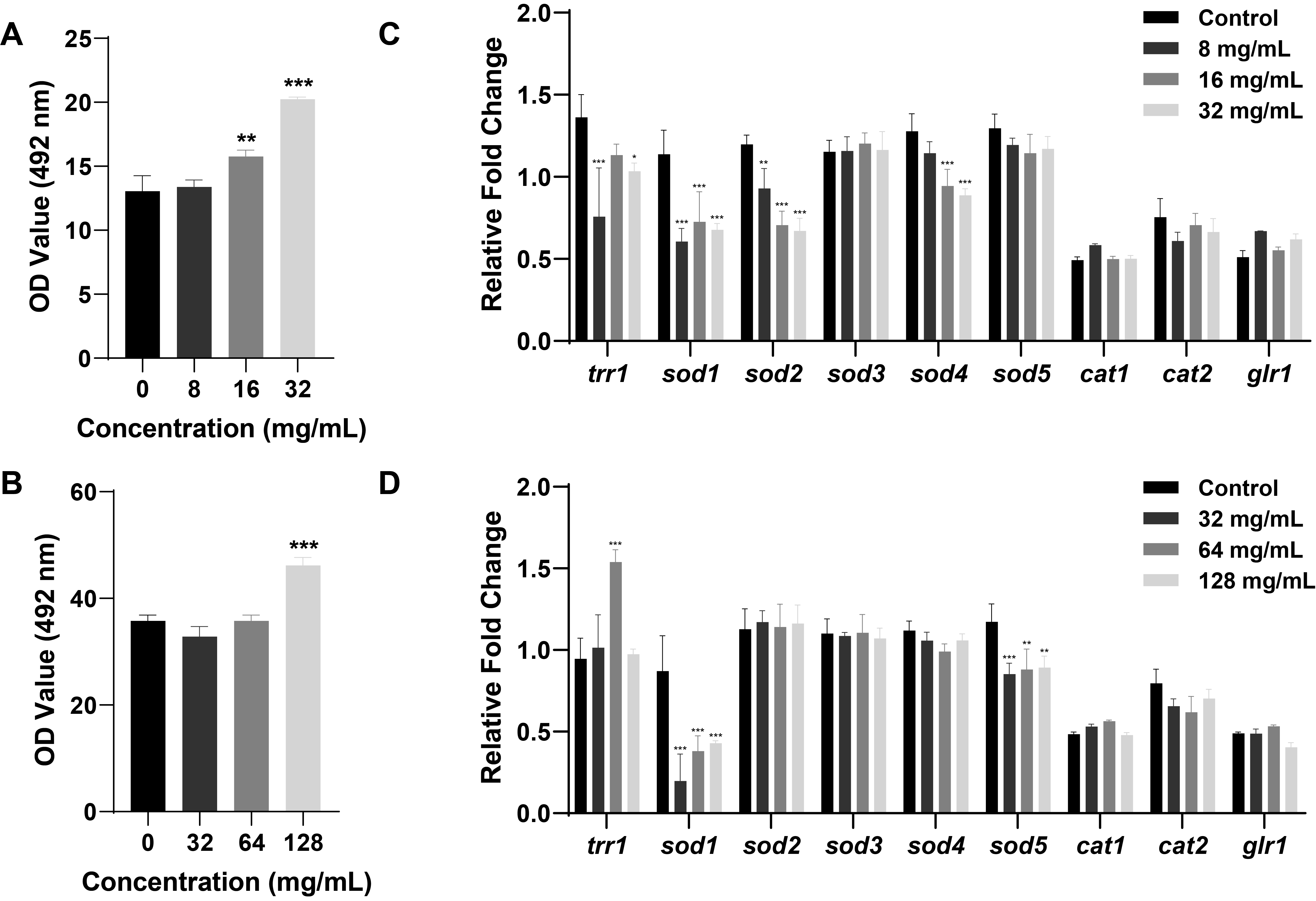
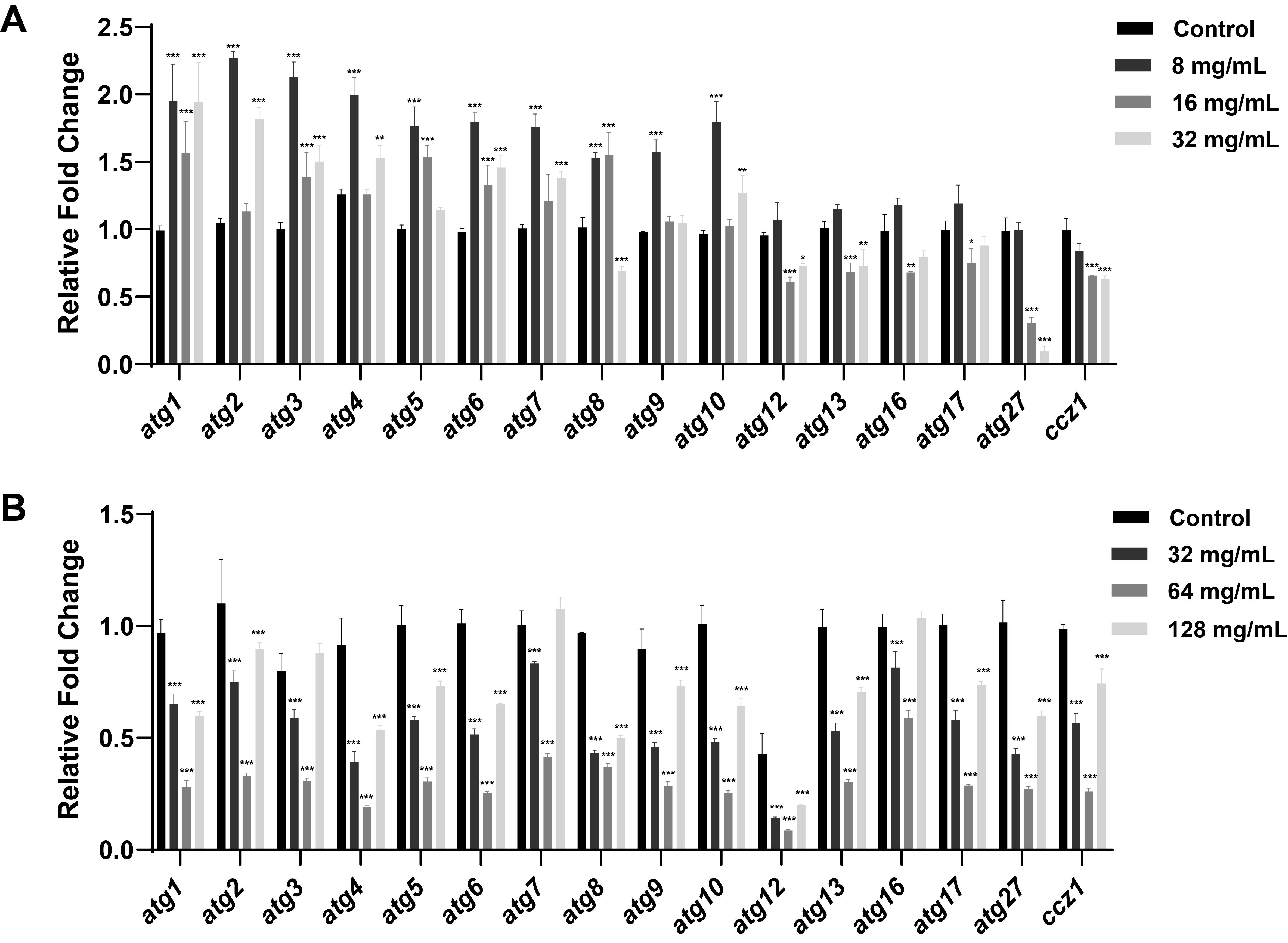
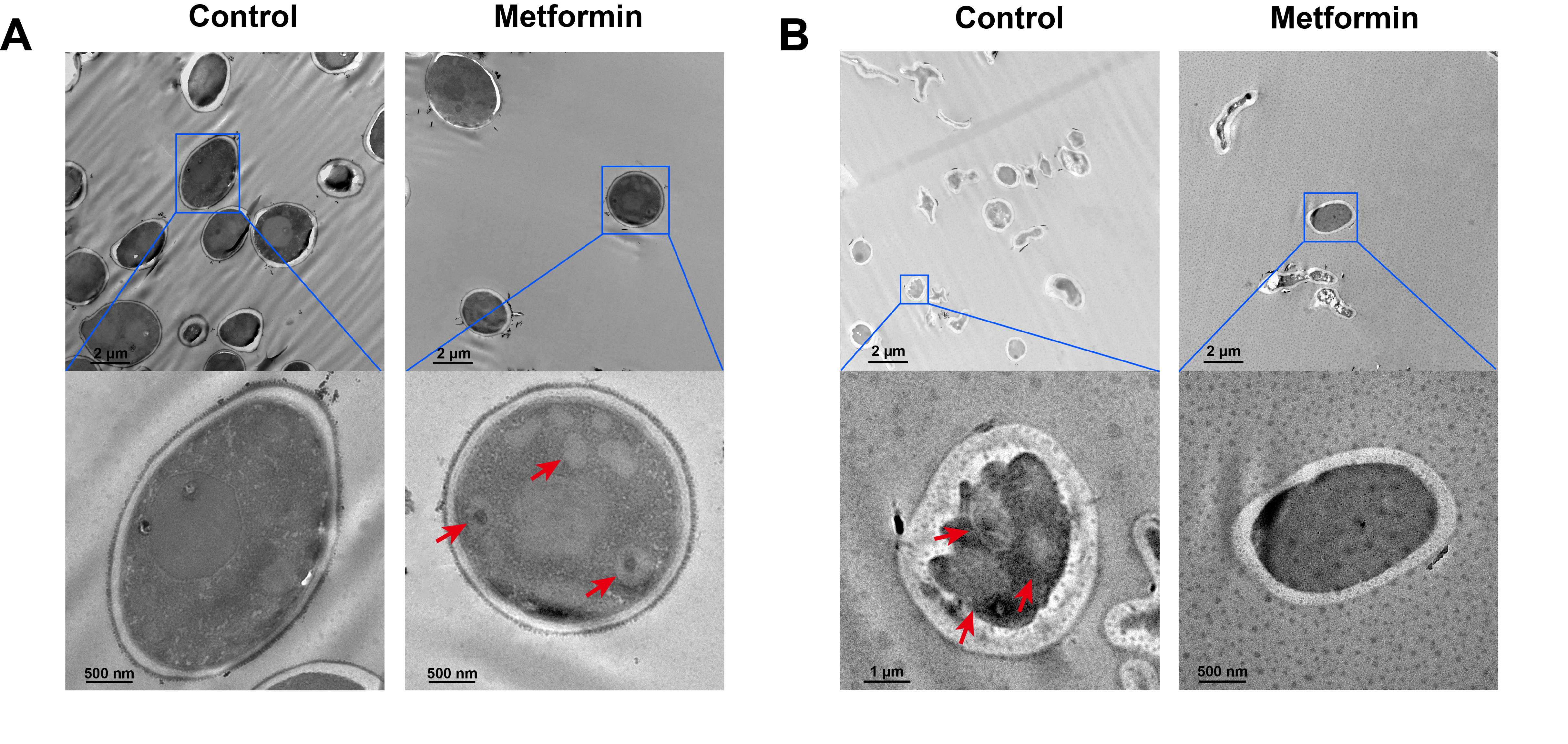
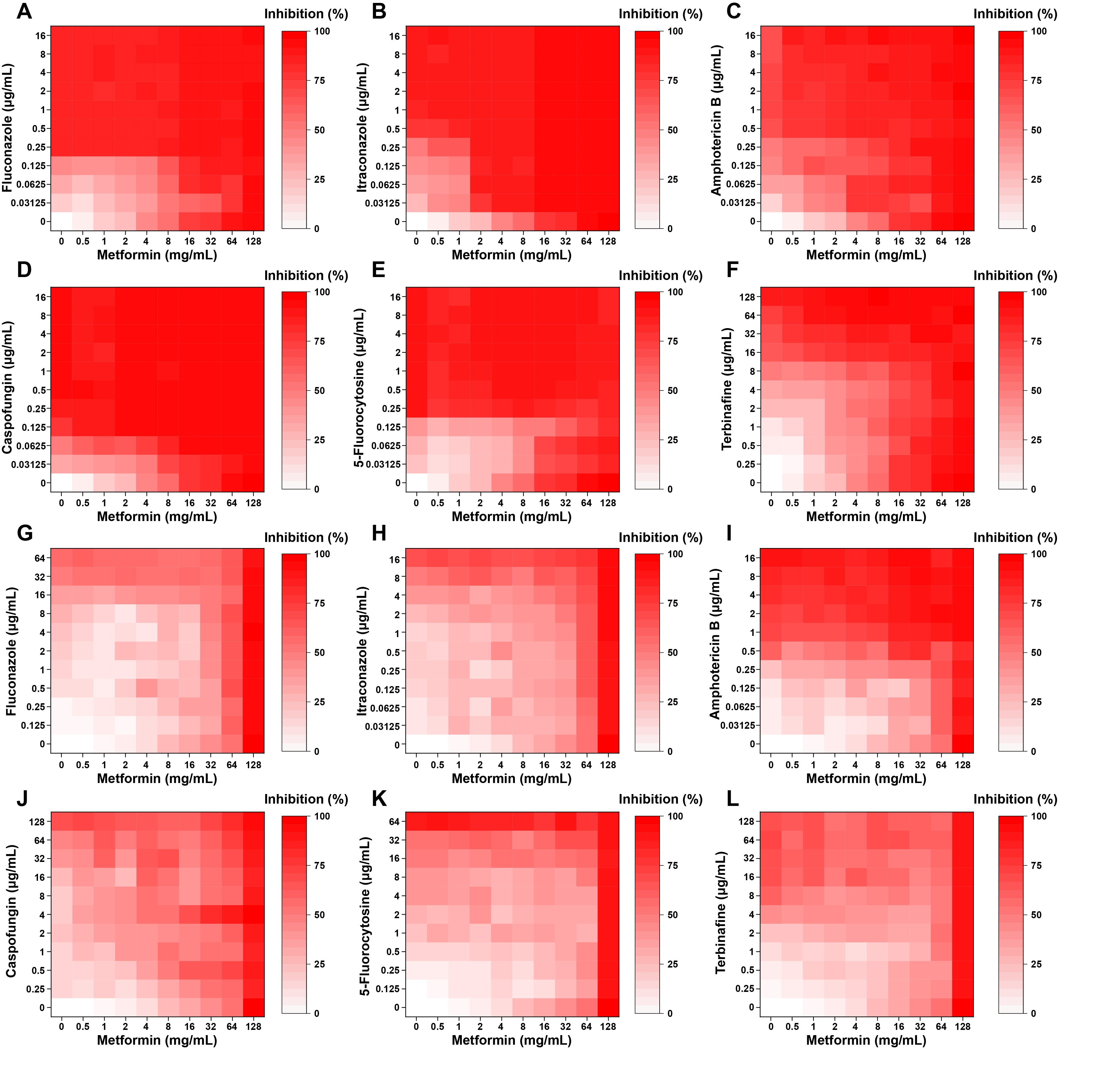
- Candida albicans (C. albicans) is a common opportunistic fungal pathogen that can cause infections ranging from superficial to severe systemic diseases. This study investigates the antifungal effects of metformin on C. albicans and explores its underlying mechanisms. Growth inhibition was assessed via XTT assays, and hyphal formation and morphological changes were observed by light microscope and scanning electron microscopy (SEM). Mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) levels were measured with JC-1 and DCFH-DA probes, respectively. Gene expression related to ROS and autophagy was quantified by RT-qPCR, and autophagosomes were visualized using transmission electron microscopy (TEM). Metformin significantly inhibited C. albicans growth and hyphal formation, altered cell morphology, reduced MMP, and increased ROS levels. It activated autophagy in planktonic C. albicans but suppressed it in biofilm forms. Additionally, metformin exhibited synergistic effects with amphotericin B against planktonic C. albicans and with caspofungin against biofilms. The findings suggest that metformin exerts antifungal activity by modulating MMP, ROS levels, and autophagy-related pathways, and enhances the efficacy of specific antifungal drugs.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant number 81970945).
Conflict of Interest
The authors declare no conflicts of interest.
Ethical Statements
This study does not involve any research with human participants or animals, and therefore ethical approval is not required.
- Abrosimov R, Baeken M, Hauf S, Wittig I, Hajieva P, et al. 2024. Mitochondrial complex I inhibition triggers NAD+-independent glucose oxidation via successive NADPH formation, "futile" fatty acid cycling, and FADH2 oxidation. GeroScience. 46(4): 3635–3658. ArticlePubMedPMCPDF
- Bagkos G, Koufopoulos K, Piperi C. 2014. A new model for mitochondrial membrane potential production and storage. Med Hypotheses. 83(2): 175–181. ArticlePubMed
- Barbarossa A, Rosato A, Carrieri A, Fumarola L, Tardugno R, et al. 2024. Exploring the antibiofilm effect of sertraline in synergy with Cinnamomum verum essential oil to counteract Candida species. Pharmaceuticals. 17(9): 1109.ArticlePubMedPMC
- Barbarossa A, Rosato A, Carrieri A, Tardugno R, Corbo F, et al. 2023. Antifungal biofilm inhibitory effects of combinations of diclofenac and essential oils. Antibiotics. 12(12): 1673.ArticlePubMedPMC
- Bekbulat F. 2023. Evolutionarily acquired response of selective autophagy receptors provides resilience against oxidative stress. Bioessays. 45(11): e2300168. ArticlePubMed
- Bren A, Glass DS, Kohanim YK, Mayo A, Alon U. 2023. Tradeoffs in bacterial physiology determine the efficiency of antibiotic killing. Proc Natl Acad Sci USA. 120(51): e2312651120. ArticlePubMedPMC
- Bridges HR, Jones AJ, Pollak MN, Hirst J. 2014. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J. 462(3): 475–487. ArticlePubMedPDF
- Carrageta DF, Freire-Brito L, Oliveira PF, Alves MG. 2022. Evaluation of human spermatozoa mitochondrial membrane potential using the JC-1 dye. Curr Protoc. 2(9): e531. ArticlePubMedLink
- Catalano L, Aminzadeh-Gohari S, Weber DD, Poupardin R, Stefan VE, et al. 2023. Triple therapy with metformin, ketogenic diet, and metronomic cyclophosphamide reduced tumor growth in MYCN-amplified neuroblastoma xenografts. Metabolites. 13(8): 910.ArticlePubMedPMC
- Chen Y, Gao Y, Li Y, Yin J. 2024. Anti-biofilm activity of assamsaponin A, theasaponin E1, and theasaponin E2 against Candida albicans. Int J Mol Sci. 25(7): 3599.ArticlePubMedPMC
- Chen J, Ou Y, Li Y, Hu J, Shao LW, et al. 2017. Metformin extends C. elegans lifespan through lysosomal pathway. eLife. 6: e31268. ArticlePubMedPMCPDF
- Clinical and Laboratory Standards Institute (CLSI). 2017. Performance standards for antimicrobial susceptibility testing. CLSI document M27-A4. Wayne, PA, USA.
- d'Enfert C, Kaune A, Alaban L, Chakraborty S, Cole N, et al. 2021. The impact of the fungus-host-microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol Rev. 45(3): fuaa060.ArticlePubMed
- Du J, Dong Y, Zhu H, Deng Y, Sa C, et al. 2023. DNA damage-induced autophagy is regulated by inositol polyphosphate synthetases in Candida albicans. Biochim Biophys Acta Mol Cell Res. 1871(1): 119622.ArticlePubMed
- Du J, Zhao H, Zhu M, Dong Y, Peng L, et al. 2022. Atg8 and Ire1 in combination regulate the autophagy-related endoplasmic reticulum stress response in Candida albicans. Res Microbiol. 174(3): 103996.ArticlePubMed
- Efentakis P, Varela A, Lamprou S, Papanagnou ED, Chatzistefanou M, et al. 2024. Implications and hidden toxicity of cardiometabolic syndrome and early-stage heart failure in carfilzomib-induced cardiotoxicity. Br J Pharmacol. 181(16): 2964–2990. ArticlePubMed
- Fang CW, Yang JS, Chiang JH, Shieh PC, Tsai FJ, et al. 2023. Metformin induces autophagy of cisplatin-resistant human gastric cancer cells in addition to apoptosis. Biomedicine (Taipei). 13(2): 14–23. ArticlePubMedPMC
- Feng Y, Zhang Y, Li J, Omran RP, Whiteway M, et al. 2022. Transcriptional profiling of the Candida albicans response to the DNA damage agent methyl methanesulfonate. Int J Mol Sci. 23(14): 7555.ArticlePubMedPMC
- Gao C, Fang L, Zhang H, Zhang WS, Li XO, et al. 2020. Metformin induces autophagy via the AMPK-mTOR signaling pathway in human hepatocellular carcinoma cells. Cancer Manag Res. 12: 5803–5811. ArticlePubMedPMC
- González A, Hall MN, Lin SC, Hardie DG. 2020. AMPK and TOR: the yin and yang of cellular nutrient sensing and growth control. Cell Metab. 31(3): 472–492. ArticlePubMed
- Kang SO, Kwak MK. 2021. Alcohol dehydrogenase 1 and NAD(H)-linked methylglyoxal oxidoreductase reciprocally regulate glutathione-dependent enzyme activities in Candida albicans. J Microbiol. 59(1): 76–91. ArticlePubMedPDF
- Kher L, Santoro D. 2023. Biofilm models: Different ways of biofilm characterization and drug discovery. Curr Protoc. 3(9): e894. ArticlePubMed
- Koshikawa T, Abe M, Nagi M, Miyazaki Y, Takemura H. 2022. Biofilm-formation capability depends on environmental oxygen concentrations in Candida species. J Infect Chemother. 28(5): 643–650. ArticlePubMed
- Krysan DJ. 2023. The mystery of Candida albicans hyphal morphogenesis in the macrophage phagolysosome. Infect Immun. 91(5): e0010423. ArticlePubMedLink
- Lee DE, Lee GY, Lee HM, Choi SY, Lee SJ, et al. 2023. Synergistic apoptosis by combination of metformin and an O-GlcNAcylation inhibitor in colon cancer cells. Cancer Cell Int. 23(1): 108.ArticlePubMedPMCPDF
- Li Y, Chang W, Zhang M, Li X, Jiao Y, et al. 2015. Synergistic and drug-resistant reversing effects of diorcinol d combined with fluconazole against Candida albicans. FEMS Yeast Res. 15(2): fov001.ArticlePubMed
- Lin J, Xiao X, Liang Y, Zhao H, Yu Y, et al. 2023. Repurposing non-antifungal drugs auranofin and pentamidine in combination as fungistatic antifungal agents against C. albicans. Front Cell Infect Microbiol. 12: 1065962.Article
- Liu Y, Jia Y, Yang K, Li R, Xiao X, et al. 2020. Metformin restores tetracyclines susceptibility against multidrug resistant bacteria. Adv Sci (Weinh). 7(12): 1902227.ArticlePubMedPMCLink
- Liu S, Jiang L, Miao H, Lv Y, Zhang Q, et al. 2022. Autophagy regulation of ATG13 and ATG27 on biofilm formation and antifungal resistance in Candida albicans. Biofouling. 38(9): 926–939. ArticlePubMed
- Lu G, Wu Z, Shang J, Xie Z, Chen C, et al. 2021. The effects of metformin on autophagy. Biomed Pharmacother. 137: 111286.ArticlePubMed
- Luther CH, Brandt P, Vylkova S, Dandekar T, Müller T, et al. 2023. Integrated analysis of Sr-like protein kinases Sky1 and Sky2 links signaling networks with transcriptional regulation in Candida albicans. Front Cell Infect Microbiol. 13: 1108235.ArticlePubMedPMC
- Lv Z, Guo Y. 2020. Metformin and its benefits for various diseases. Front Endocrinol. 11: 191.Article
- Mathé L, Van Dijck P. 2013. Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet. 59(4): 251–264. ArticlePubMedPMCPDF
- Miceli C, Leri M, Stefani M, Bucciantini M. 2023. Autophagy-related proteins: Potential diagnostic and prognostic biomarkers of aging-related diseases. Ageing Res Rev. 89: 101967.ArticlePubMed
- Moraes DC, Ferreira-Pereira A. 2019. Insights on the anticandidal activity of non-antifungal drugs. J Mycol Med. 29(3): 253–259. ArticlePubMed
- Osset-Trénor P, Pascual-Ahuir A, Proft M. 2023. Fungal drug response and antimicrobial resistance. J Fungi (Basel). 9(5): 565.ArticlePubMedPMC
- Pereira R, Dos Santos Fontenelle RO, de Brito EHS, de Morais SM. 2021. Biofilm of Candida albicans: Formation, regulation and resistance. J Appl Microbiol. 131(1): 11–22. ArticlePubMedLink
- Podhorecka M, Ibanez B, Dmoszyńska A. 2017. Metformin - its potential anti-cancer and anti-aging effects. Postepy Hig Med Dosw (Online). 71: 170–175. ArticlePubMedLink
- Rabaan AA, Sulaiman T, Al-Ahmed SH, Buhaliqah ZA, Buhaliqah AA, et al. 2023. Potential strategies to control the risk of antifungal resistance in humans: A comprehensive review. Antibiotics. 12(3): 608.ArticlePubMedPMC
- Sengking J, Mahakkanukrauh P. 2024. The underlying mechanism of calcium toxicity-induced autophagic cell death and lysosomal degradation in early stage of cerebral ischemia. Anat Cell Biol. 57(2): 155–162. ArticlePubMedPMC
- Sharma D, Misba L, Khan AU. 2019. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob Resist Infect Control. 8: 76.ArticlePubMedPMCPDF
- Shen J, Ma M, Duan W, Huang Y, Shi B, et al. 2023. Autophagy alters the susceptibility of Candida albicans biofilms to antifungal agents. Microorganisms. 11(8): 2015.ArticlePubMedPMC
- Sturaro MC, de Souza GHA, Damaceno NDS, Silva ON, de Aquino TM, et al. 2024. Antimicrobial activity of ceftibuten/polymyxin B combination against polymyxin/carbapenem-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 80(1): 116–125. ArticlePDF
- Sun X, Ping J, Guo X, Long J, Cai Q, et al. 2024. Drug-target mendelian randomization revealed a significant association of genetically proxied metformin effects with increased prostate cancer risk. Mol Carcinog. 63(5): 849–858. ArticlePubMedPMC
- Todd RT, Soisangwan N, Peters S, Kemp B, Crooks T, et al. 2023. Antifungal drug concentration impacts the spectrum of adaptive mutations in Candida albicans. Mol Biol Evol. 40(1): msad009.ArticlePubMedPMCPDF
- Vyas HKN, Xia B, Mai-Prochnow A. 2022. Clinically relevant in vitro biofilm models: A need to mimic and recapitulate the host environment. Biofilm. 4: 100069.ArticlePubMedPMC
- Wall G, Montelongo-Jauregui D, Vidal Bonifacio B, Lopez-Ribot JL, Uppuluri P. 2019. Candida albicans biofilm growth and dispersal: Contributions to pathogenesis. Curr Opin Microbiol. 52: 1–6. ArticlePubMedPMC
- Wójcik-Mieszawska S, Lewtak K, Skwarek E, Dębowski D, Gitlin-Domagalska A, et al. 2023. Autophagy of Candida albicans cells after the action of earthworm venetin-1 nanoparticle with protease inhibitor activity. Sci Rep. 13(1): 14228.ArticlePubMedPMC
- Xu S, Feliu M, Lord AK, Lukason DP, Negoro PE, et al. 2018. Biguanides enhance antifungal activity against Candida glabrata. Virulence. 9(1): 1150–1162. ArticlePubMedPMC
- Yang M, Darwish T, Larraufie P, Rimmington D, Cimino I, et al. 2021. Inhibition of mitochondrial function by metformin increases glucose uptake, glycolysis and GDF-15 release from intestinal cells. Sci Rep. 11(1): 2529.ArticlePubMedPMCPDF
- Yang HD, Zhu QF, Li H, Jiang XL, Xu XQ, et al. 2022. Effect of Neiyi Prescription of QIU on autophagy and angiogenic ability of endometriosis via the PPARγ/NF-κB signaling pathway. Arch Gynecol Obstet. 306(2): 533–545. ArticlePubMedPDF
- Yu LH, Wei X, Ma M, Chen XJ, Xu SB. 2011. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm. Antimicrob Agents Chemother. 56(2): 770–775. ArticlePubMedLink
- Zhang Y, Kepiro I, Ryadnov MG, Pagliara S. 2023. Single cell killing kinetics differentiate phenotypic bacterial responses to different antibacterial classes. Microbiol Spectr. 11(1): e0366722. ArticlePubMedLink
- Zhao B, Luo J, Wang Y, Zhou L, Che J, et al. 2019. Metformin suppresses self-renewal ability and tumorigenicity of osteosarcoma stem cells via reactive oxygen species-mediated apoptosis and autophagy. Oxid Med Cell Longev. 2019: 9290728.ArticlePubMedPMCPDF
- Zhao MM, Wang B, Huang WX, Zhang L, Peng R, et al. 2024. Verteporfin suppressed mitophagy via PINK1/parkin pathway in endometrial cancer. Am J Cancer Res. 14(4): 1935–1946. ArticlePubMedPMC
- Zhou Y, Wang G, Li Y, Liu Y, Song Y, et al. 2012. In vitro interactions between aspirin and amphotericin B against planktonic cells and biofilm cells of Candida albicans and C. parapsilosis. Antimicrob Agents Chemother. 56(6): 3250–3260. ArticlePubMedPMCLink
References
Figure & Data
References
Citations

- Chloroquine Alone and Combined with Antifungal Drug Against Candida albicans Biofilms In Vitro and In Vivo via Autophagy Inhibition
Xiao Zhao, Qiaochu Wu, Chenyu Weng, Shuangbo Xu, Yufei Wang, Weiyu Yuan, Xuening Xiong, Wanjing Chen, Xin Wei
Mycopathologia.2025;[Epub] CrossRef -
Updates on
Candida albicans
infections: pathogenesis, resistance, and emerging nanopharmaceutical strategies
Marilena Pariano, Matteo Puccetti, Consuelo Fabi, Emilia Nunzi, Sarah Balucchi, Luana Perioli, Maurizio Ricci, Stefano Giovagnoli, Enrico Garaci, Luigina Romani
Expert Review of Anti-infective Therapy.2025; 23(10): 951. CrossRef
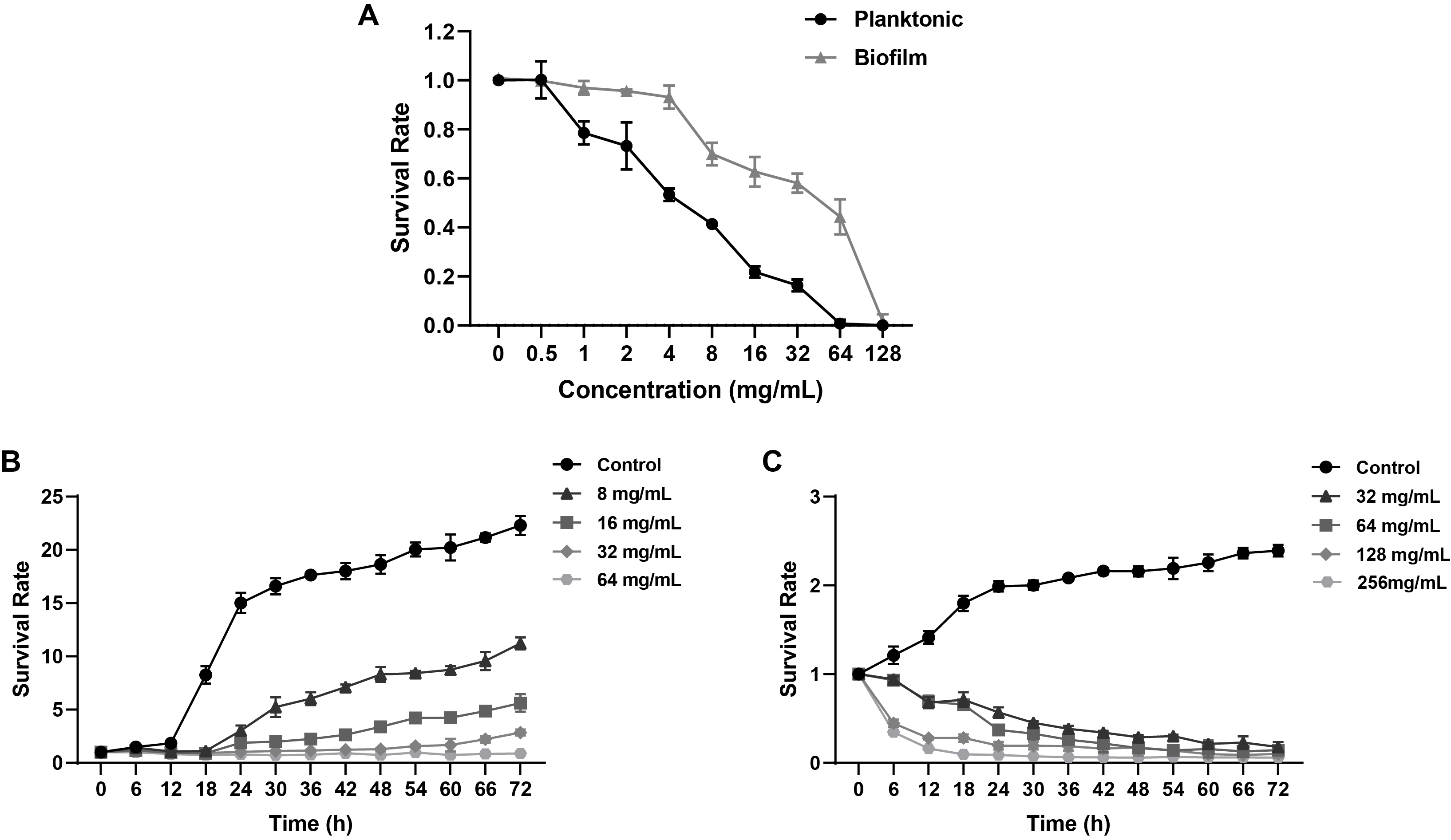
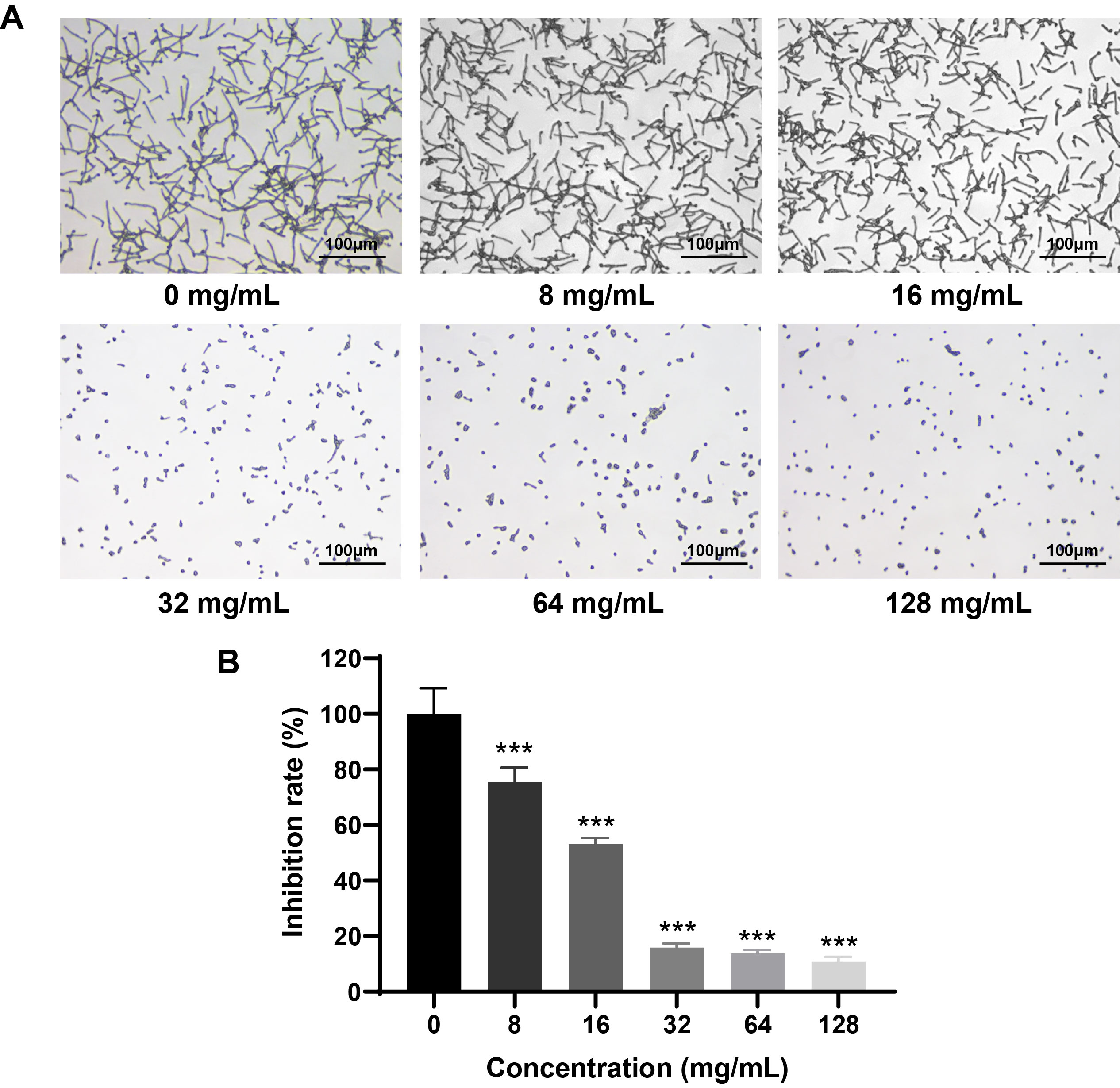
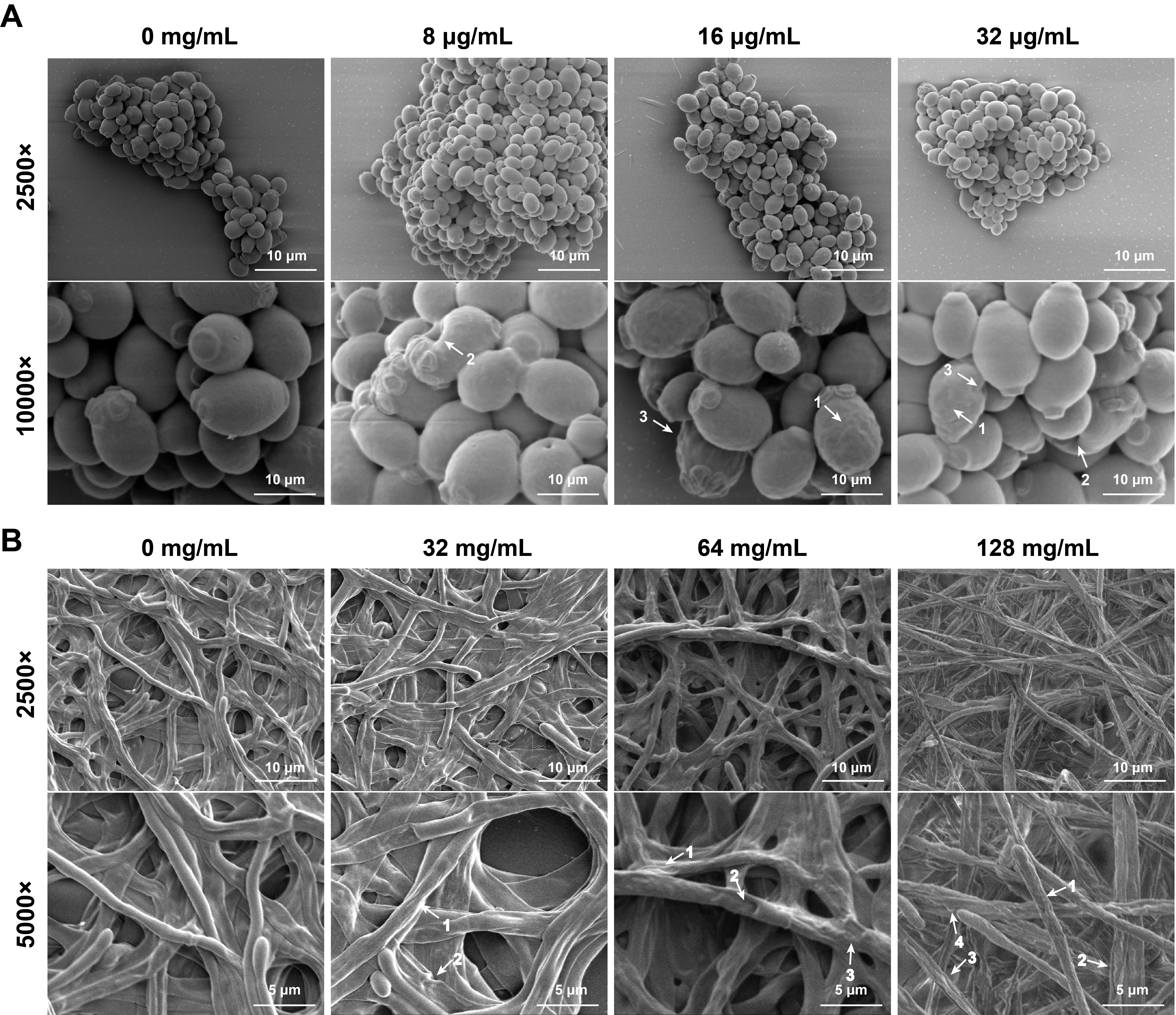
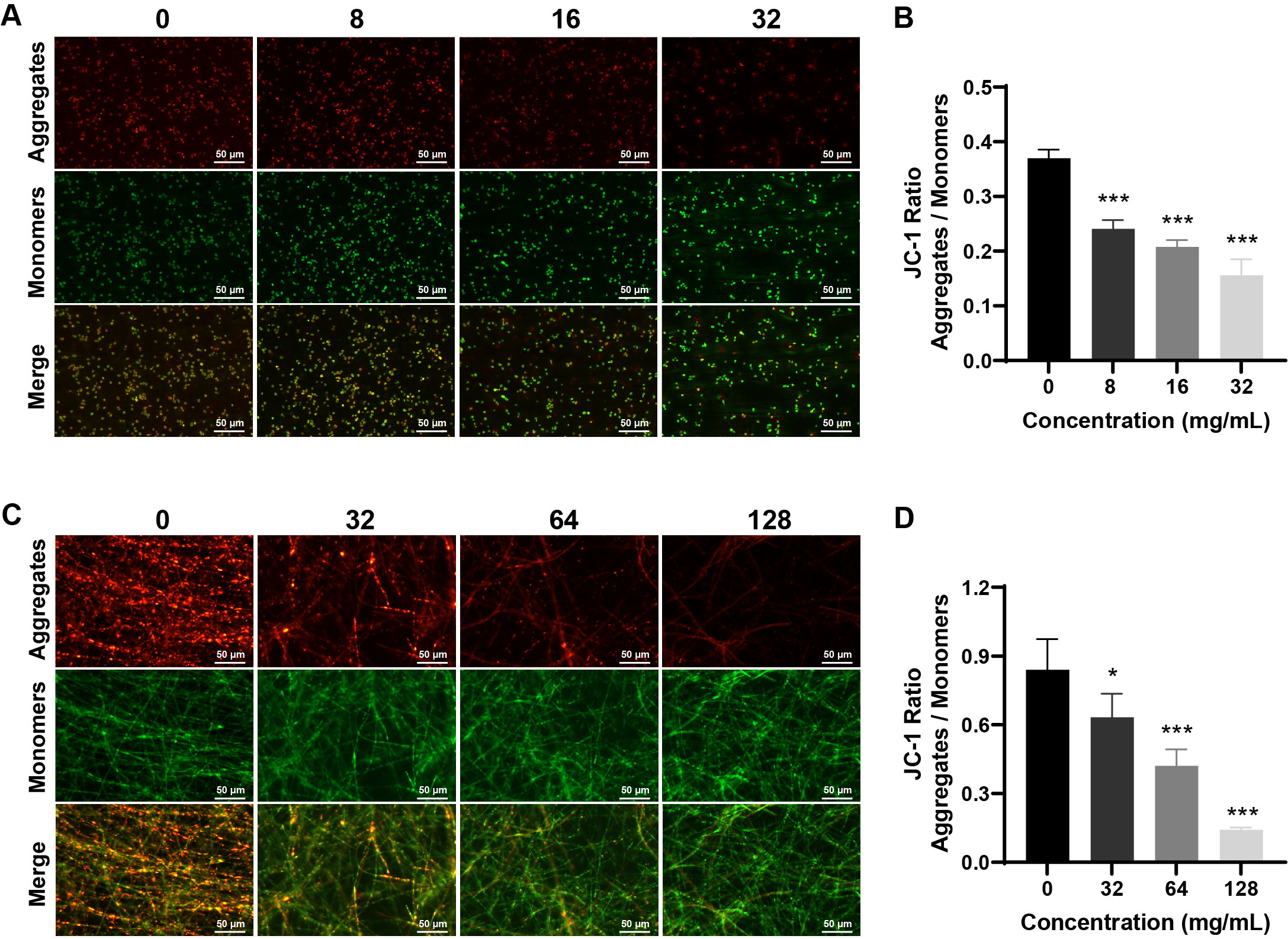
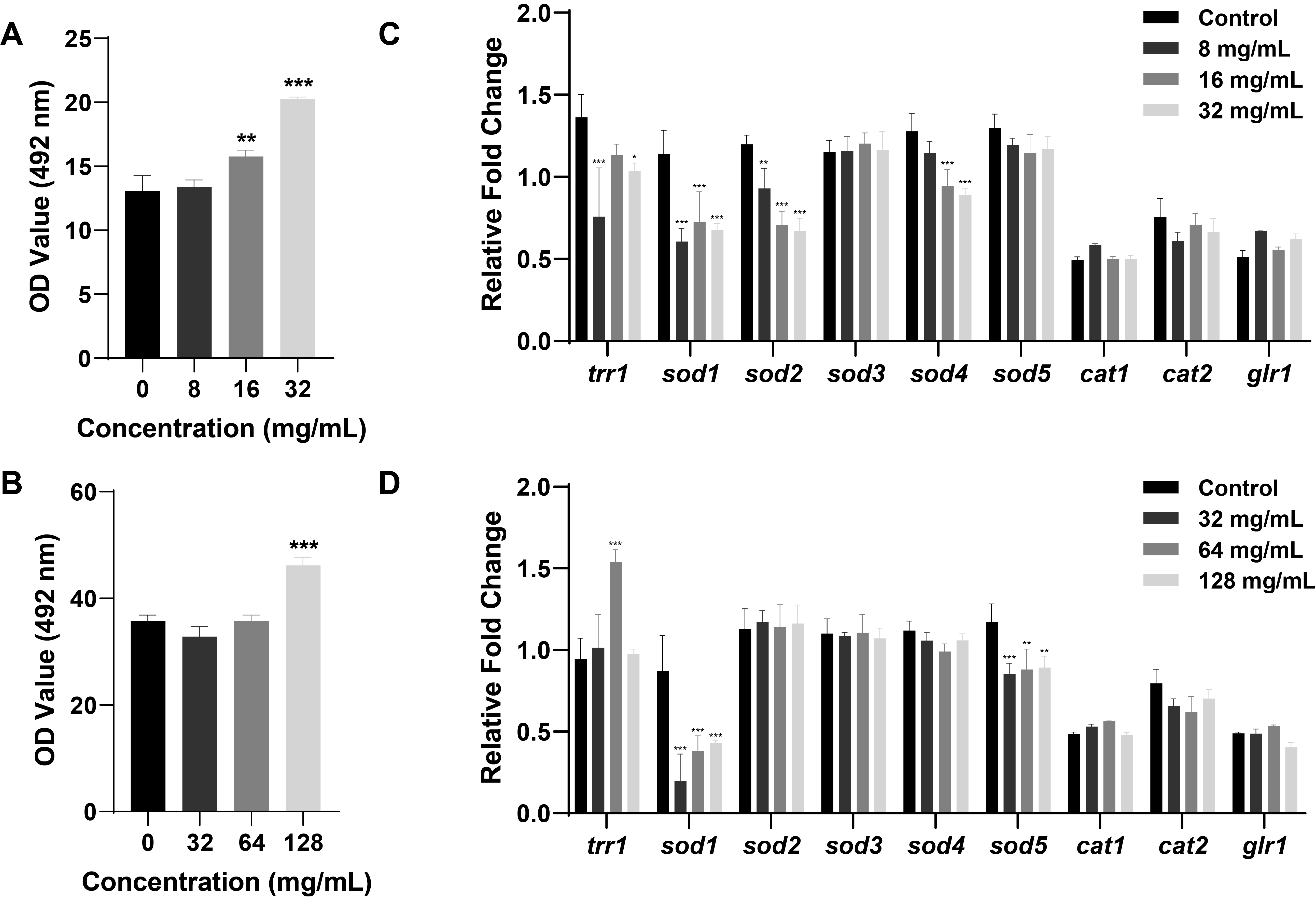
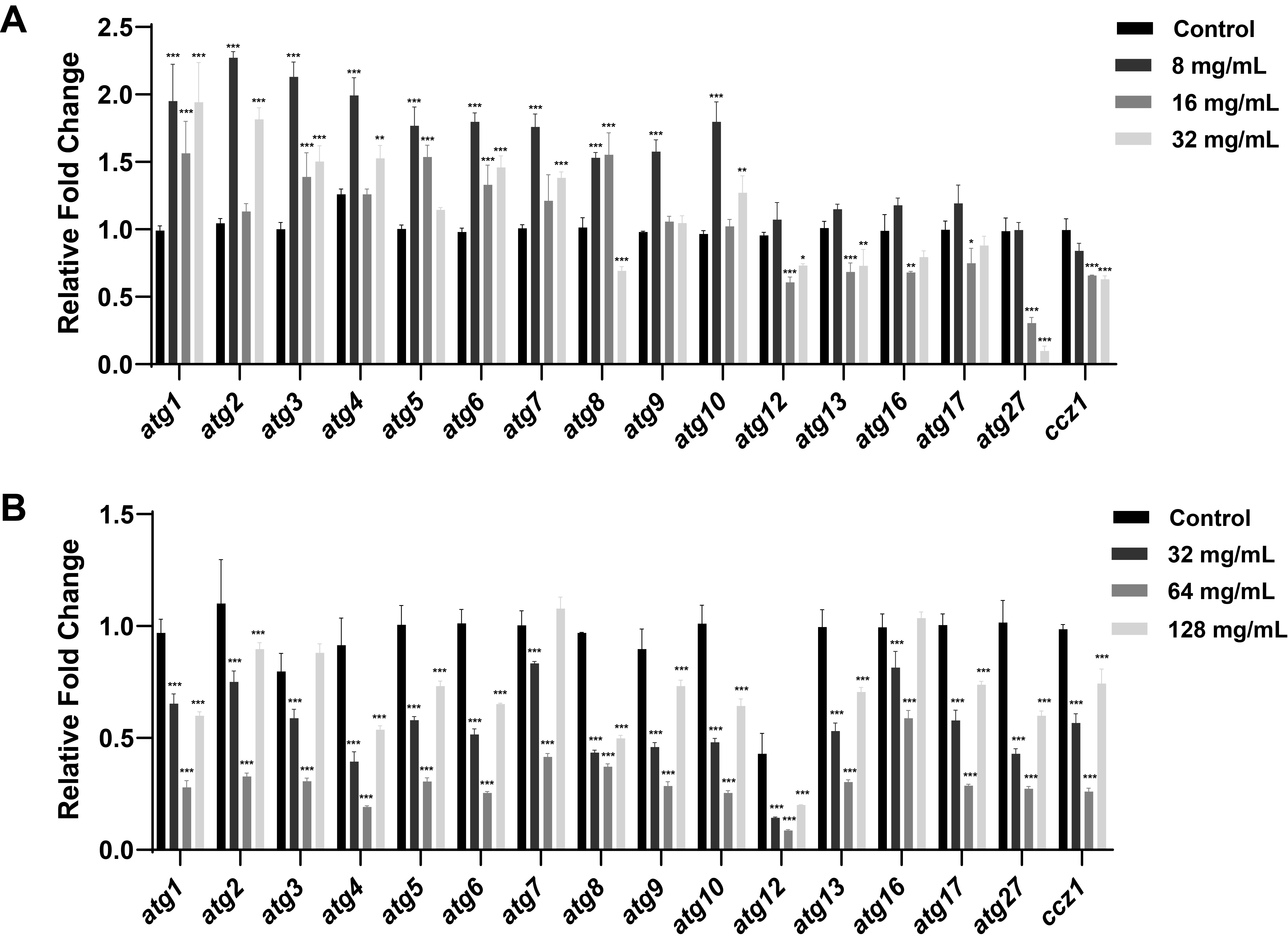
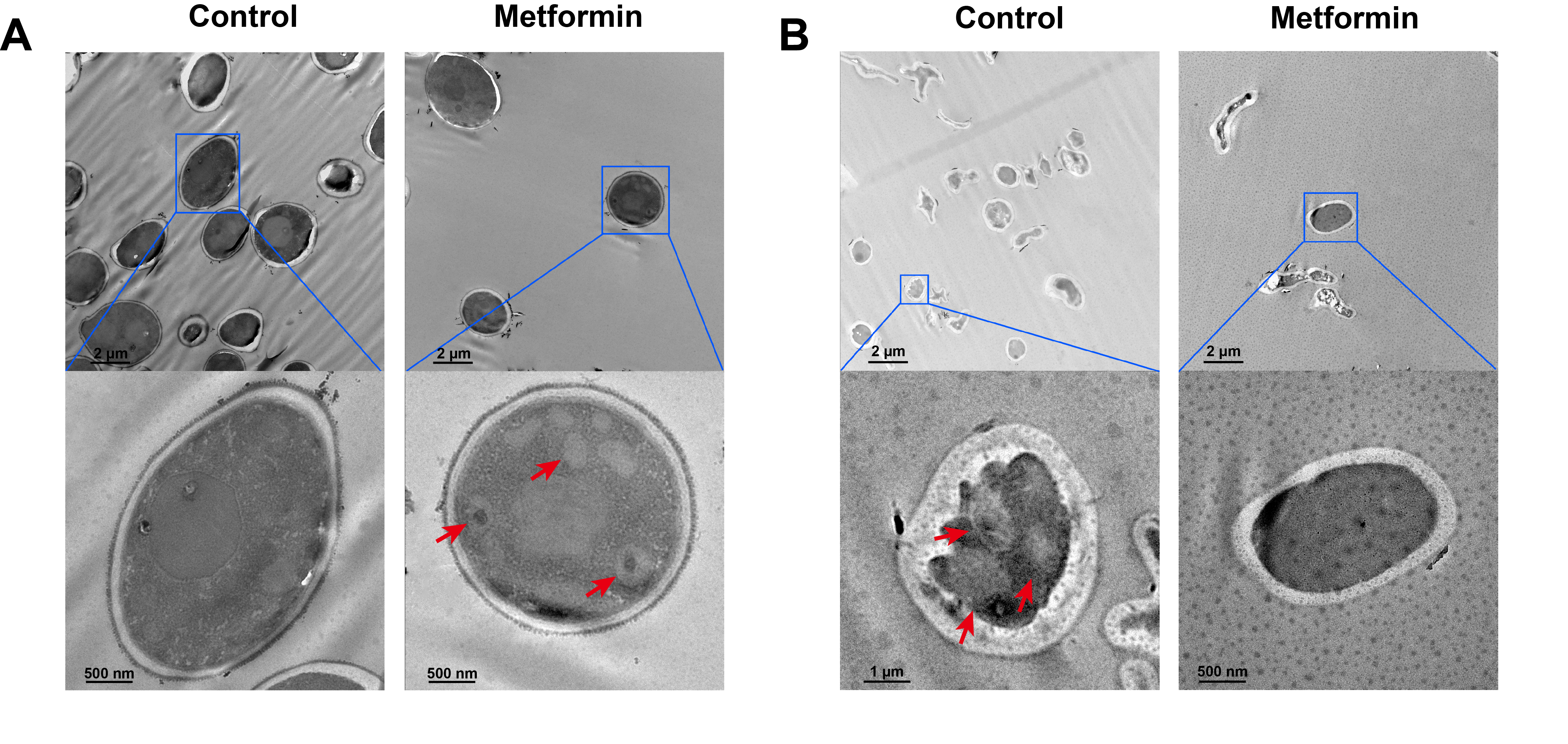
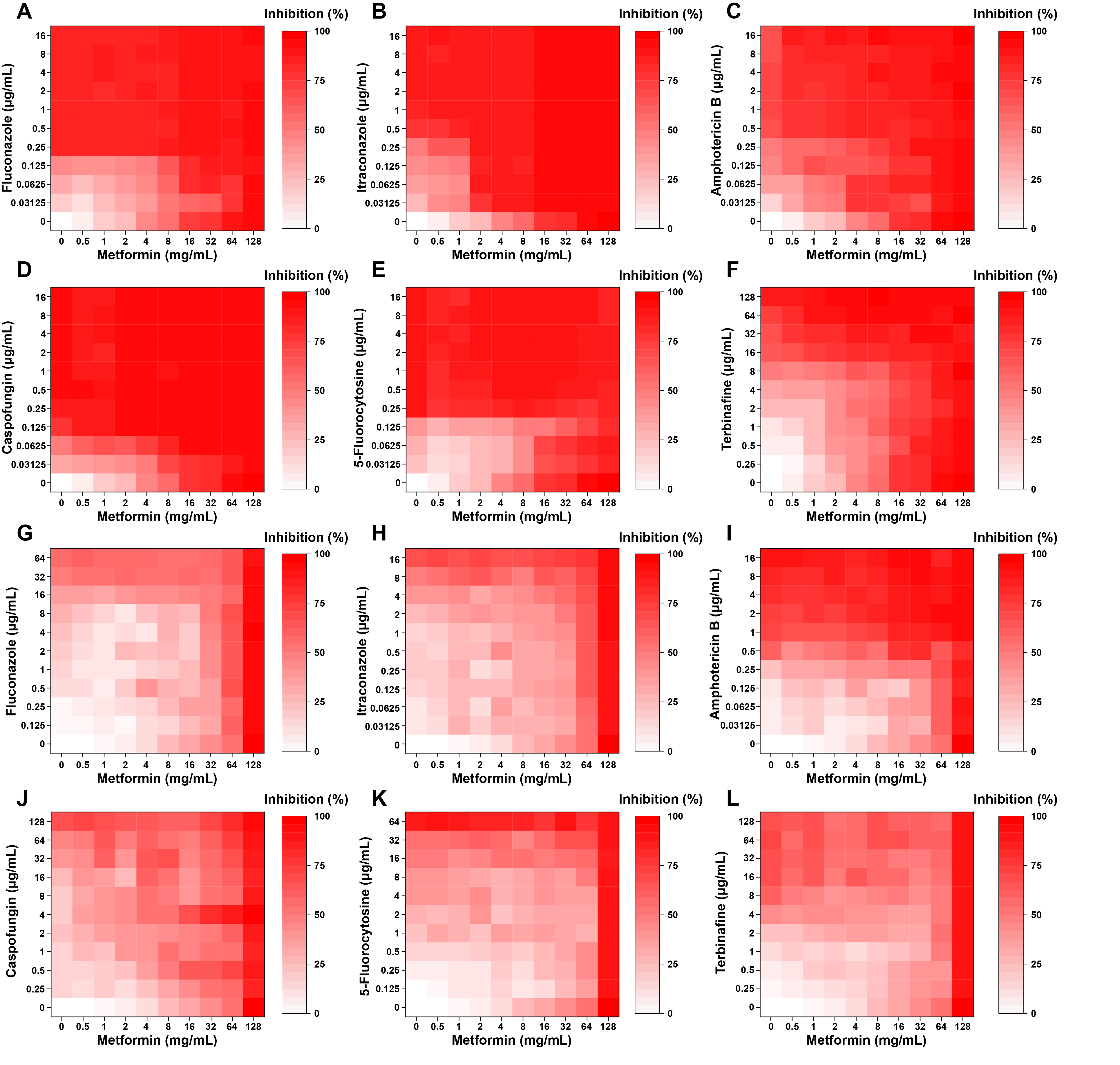
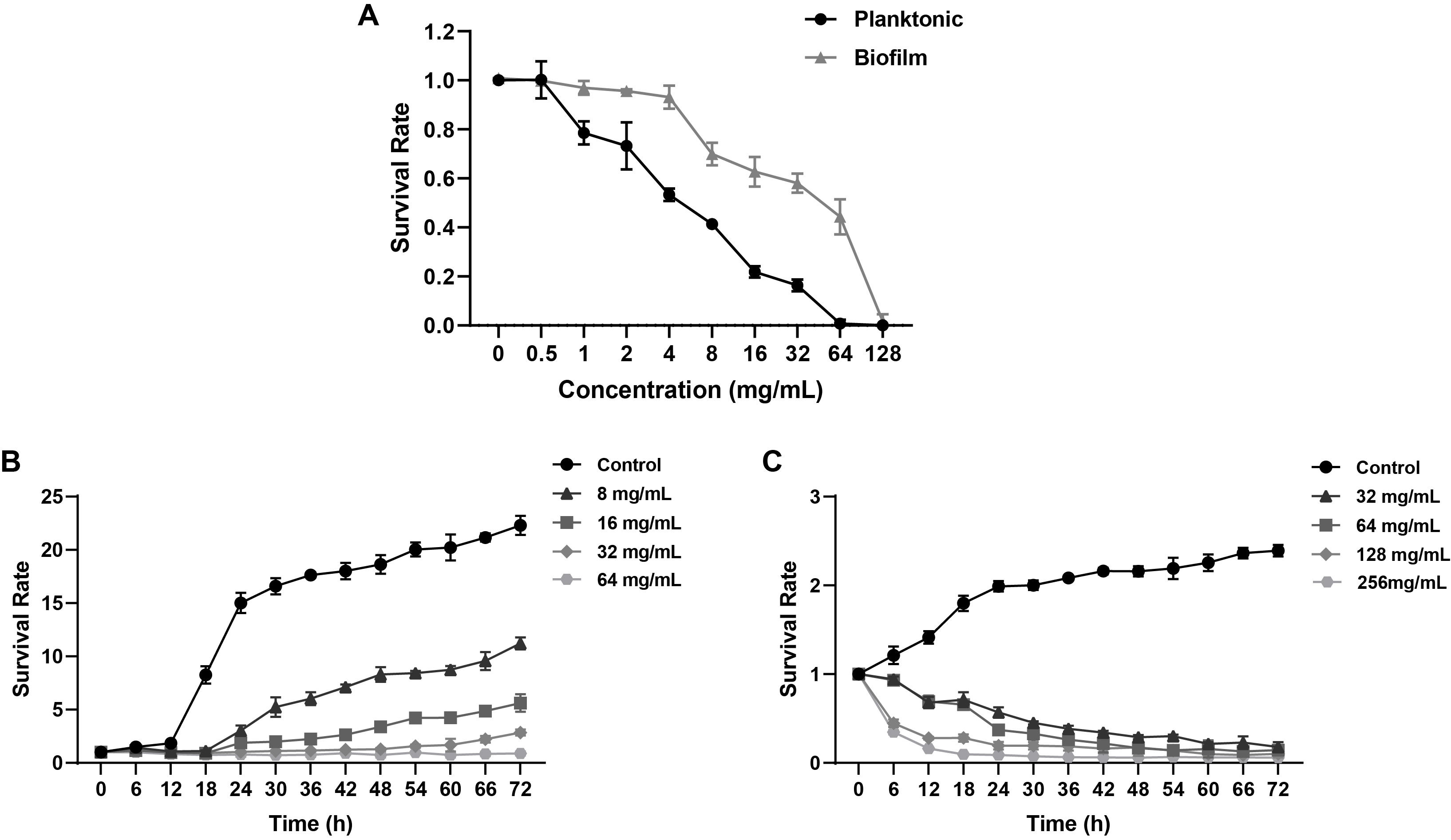
Fig. 1.
Fig. 2.
Fig. 3.
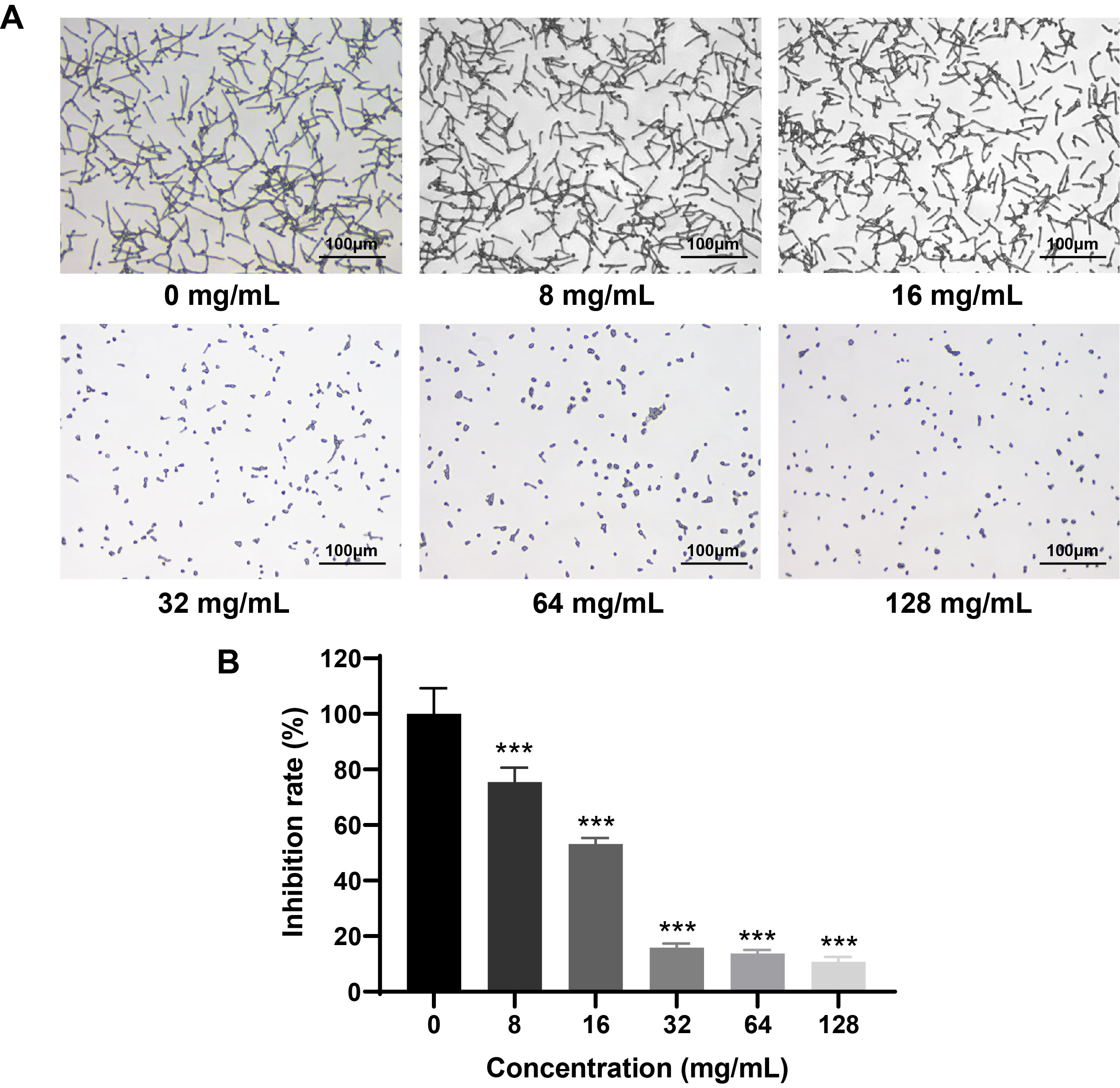
Fig. 4.
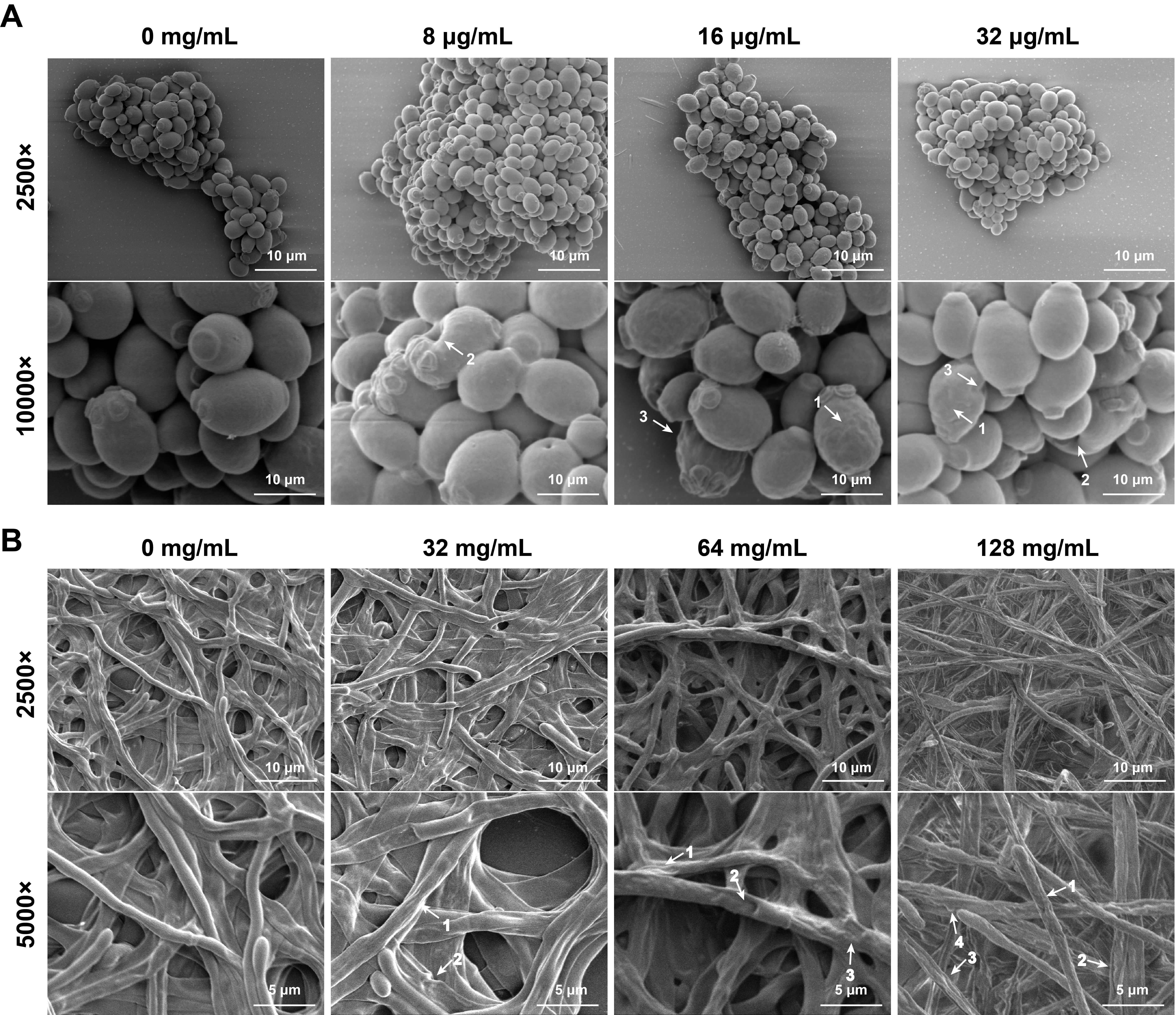
Fig. 5.
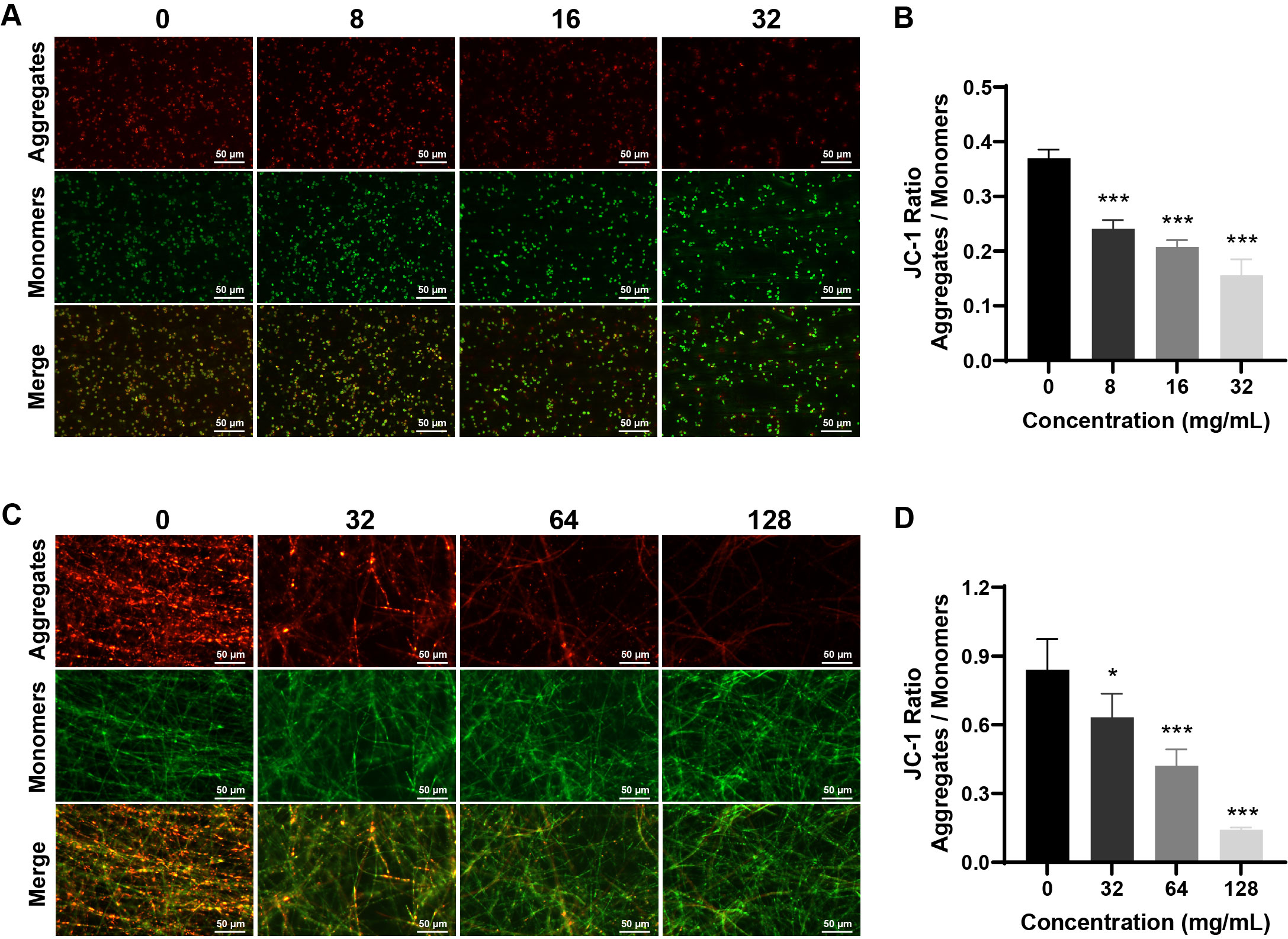
Fig. 6.
Fig. 7.
Fig. 8.
| Genes | Sequence (5’→3’) |
|---|---|
| trr1 | F: GACCAACTCAAGACCGACGAAGC |
| R: GCCATACATCCACTACCAGCAGAAG | |
| sod1 | F: ACAAGAATCCGAATCCGCTCCAAC |
| R: AGGACCAGCAGAAGTACAACCATTG | |
| sod2 | F: GAACAAGCCGTTGAAGCCAA |
| R: ACCTTGAGAGACAGGAGCCA | |
| sod3 | F: CCAAGGATCAGGTTGGGCAT |
| R: AGTACGCATGTTCCCAAGCA | |
| sod4 | F: ACGATACTGCAAGTGCTGCT |
| R: CAGCACCGCTACCTTGAGAA | |
| sod5 | F: ACTTTGCTTGACGAGGGACA |
| R: CAGCGCCATTACCTTGAGGA | |
| cat1 | F: CCAATTCCAGAACCATTTGCCACTC |
| R: ACCATAAGCACCGGAACCTTTAGC | |
| cat2 | F: TCAAGAATGGACGCCACACC |
| R: TTCTTCATCGTGGGCAGCAA | |
| glr1 | F: TGACAAGACTTTGATCGCCACTGG |
| R: TCCAAGGCAAAGAACCCATCAGATG | |
| β-actin | F: GACCAAGAAGACATCAAGGTATCAT |
| R: GTGTTCAATTGGGTATCTCAAG |
| Genes | Sequence (5’→3’) |
|---|---|
| atg1 | F: TACAACCCAACTGAGCGGAT |
| R: GTAGTGGGTGATGGGCTTCT | |
| atg2 | F: GCCAAGACTACGGGGTATGA |
| R: GCCAAGACTACGGGGTATGA | |
| atg3 | F: AACGTGGCAATGGGGTAATG |
| R: CGTCCTCCTCTTCCTCTTCC | |
| atg4 | F: AGTGGTAGAGACGCCAATCA |
| R: GCACCGGTAATGTATGTGGG | |
| atg5 | F: CATGACTTGGGTTGCTGGAC |
| R: TGTTGGCTTCACTCAATTGCA | |
| atg6 | F: GCCAGAAATCAATGCCGCAT |
| R: CCATCTACTGCATCCTTGGC | |
| atg7 | F: CTGGGGTGTCAGGAGCATTA |
| R: GCATCTACACCGGGGAAAAC | |
| atg8 | F: GCCAGAATTGCTCAGAGG |
| R: GATTAATGCAGCGGTTGG | |
| atg9 | F: TACCGCAACACCAACAACAA |
| R: CTCCGTTTCAATTGGGGTGG | |
| atg10 | F: GAACGTTGGCAGTTGGGATT |
| R: AACCCGTATAGCCCAAACCA | |
| atg12 | F: ACAAGACCCAATCCCAACCA |
| R: TGGTTGAATCGACGGTGTTG | |
| atg13 | F: AGTGTCCCGTCGTCTTCA |
| R: ATGGAATCCTCATGACCCG | |
| atg16 | F: CCTTTTGGGACATCATTCTTCG |
| R: GCGCATCGAAGACATACGA | |
| atg17 | F: CCATCGGAGTTCAAGCTTCC |
| R: TCCGGTGATCATGTCCATCA | |
| atg27 | F: GCCACCTTCGCAAACAAA |
| R: TGAAACCAAGCACCACCA | |
| ccz1 | F: TGTCTCCCAAAGCCGAATCT |
| R: GTTGTTGTGGACGTGGTTGA |
| Drug combination with metformin | Planktonic |
Biofilm |
||
|---|---|---|---|---|
| FICI | Interpretation | FICI | Interpretation | |
| Fluconazole | 2 | IND | 1 | ADD |
| Itraconazole | 0.75 | ADD | 0.75 | ADD |
| Amphotericin B | 0.375 | SYN | 1 | ADD |
| Caspofungin | 1.5 | IND | 0.125 | SYN |
| 5-Fluorocytosine | 3 | ANT | 1.5 | IND |
| Terbinafine | 1.125 | IND | 1.25 | IND |
SYN: synergism; ADD: additivity; IND: indifference; ANT: antagonism
Table 1.
Table 2.
Table 3.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article