Articles
- Page Path
- HOME > J. Microbiol > Volume 63(4); 2025 > Article
-
Full article
The key pathways and genes related to oncolytic Newcastle disease virus-induced phenotypic changes in ovarian cancer cells - Wei Song, Yuan Yuan, Fangfang Cao, Huazheng Pan*, Yaqing Liu*
-
Journal of Microbiology 2025;63(4):e2411018.
DOI: https://doi.org/10.71150/jm.2411018
Published online: April 29, 2025
Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University, Qingdao 266000, P. R. China
- *Correspondence Huazheng Pan panhuazheng@126.com Yaqing Liu liuyq0532@qq.com
• Received: November 12, 2024 • Revised: January 20, 2025 • Accepted: January 24, 2025
© The Microbiological Society of Korea
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- The poor prognosis and high recurrence rate of ovarian cancer highlight the urgent need to develop new therapeutic strategies. Oncolytic Newcastle disease virus (NDV) can kill cancer cells directly and regulate innate and adaptive immunity. In this study, ovarian cancer cells infected with or without velogenic NDV-BJ were subjected to a CCK-8 assay for detecting cell proliferation, flow cytometry for detecting the cell cycle and apoptosis, and wound healing and transwell assays for detecting cell migration and invasion. Transcriptomic sequencing was conducted to identify the differentially expressed genes (DEGs). GO and KEGG enrichment analyses were performed to explore the mechanism underlying the oncolytic effect of NDV on ovarian cancer cells. The results showed that infection with NDV inhibited ovarian cancer cell proliferation, migration, and invasion; disrupted the cell cycle; and promoted apoptosis. Compared with those in negative control cells, the numbers of upregulated and downregulated genes in ovarian cancer cells infected with NDV were 1,499 and 2,260, respectively. Thirteen KEGG pathways related to cell growth and death, cell mobility, and signal transduction were significantly enriched. Among these pathways, 48 DEGs, especially SESN2, HLA B/C/E, GADD45B, and RELA, that may be involved in the oncolytic process were screened, and qPCR analysis verified the reliability of the transcription data. This study discovered some key pathways and genes related to oncolytic NDV-induced phenotypic changes in ovarian cancer cells, which will guide our future research directions and help further explore the specific mechanisms by which infection with NDV suppresses ovarian cancer development.
Introduction
Materials and Methods
Results
Discussion
Acknowledgments
This work was supported by a grant from the Natural Science Foundation of Shandong Province, China (No. ZR2023QC295).
Conflict of Interest
The authors declare that they have no competing interests.
Supplementary Information
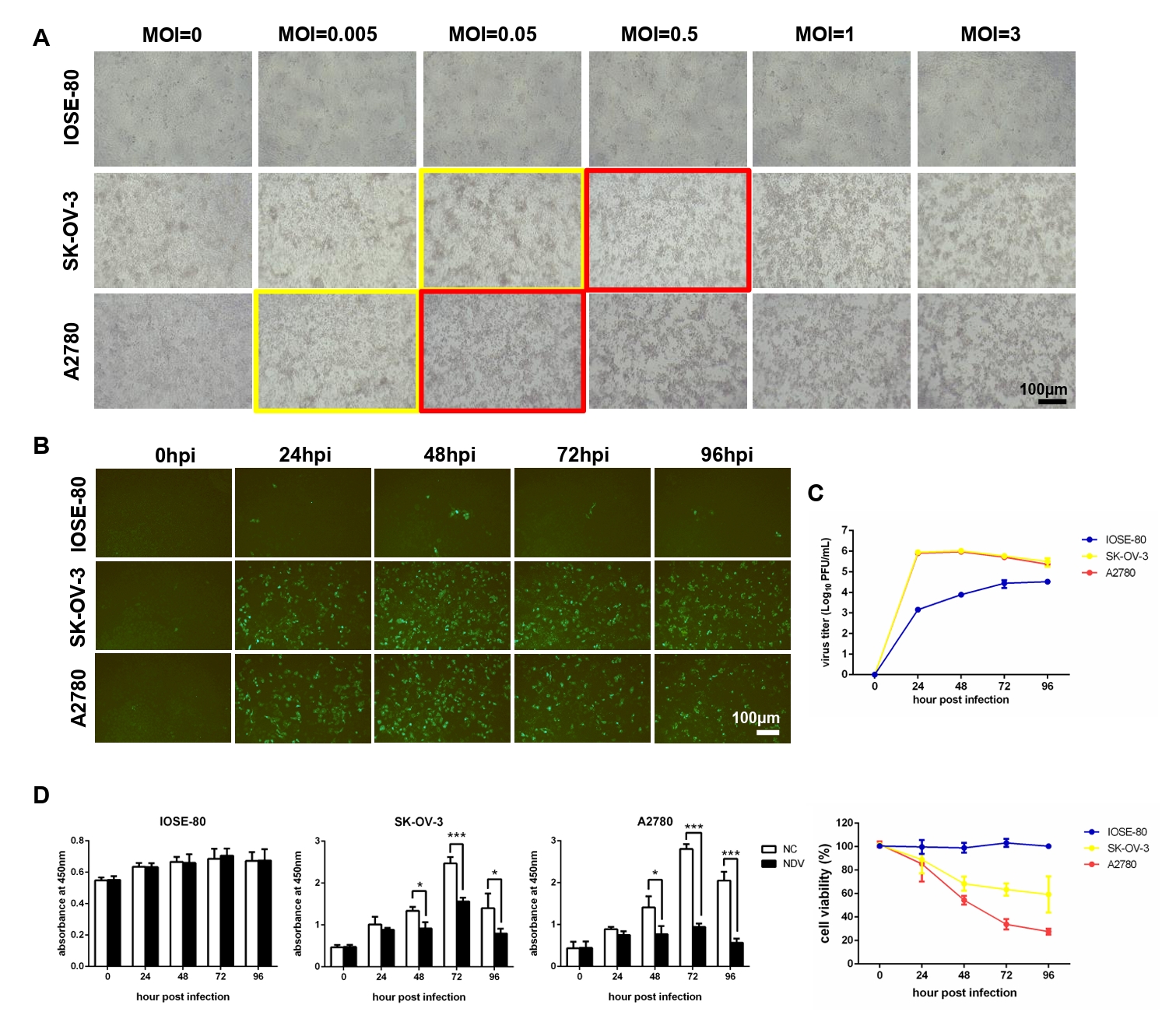
Fig. 1.NDV can grow speedily in ovarian cancer cells and suppress cell proliferation. (A) Determination of the optimal MOI of NDV by observing the CPE. The yellow rectangles indicate the onset of CPE and the red rectangles indicate the obvious CPE. The multi-step growth of NDV was detected by IFA (B) and plaque assay (C). (D) The cell proliferation was detected by CCK-8 assay (*, P < 0.05; ***, P < 0.001; otherwise, P > 0.05).
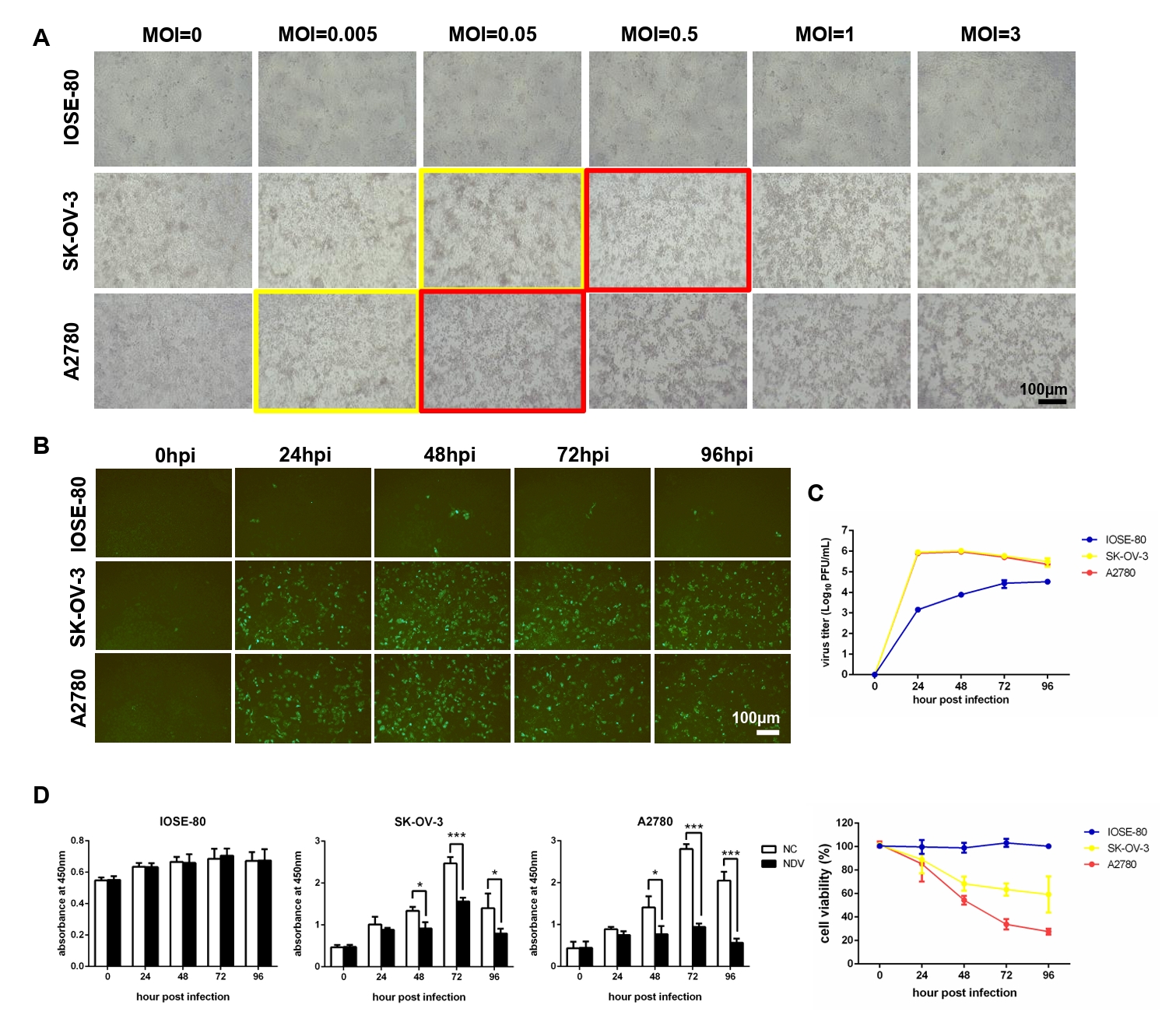

Fig. 2.Infection with NDV caused a disrupted cell cycle and prompted apoptosis of ovarian cancer cells. Flow cytometry was performed to explore the influence of NDV on the cell cycle (A) and apoptosis (B) of ovarian cancer cells. The flow cytometry on the left displays a representative experimental result, while the histogram on the right shows the average results of three experiments (***, P < 0.001; otherwise, P > 0.05).
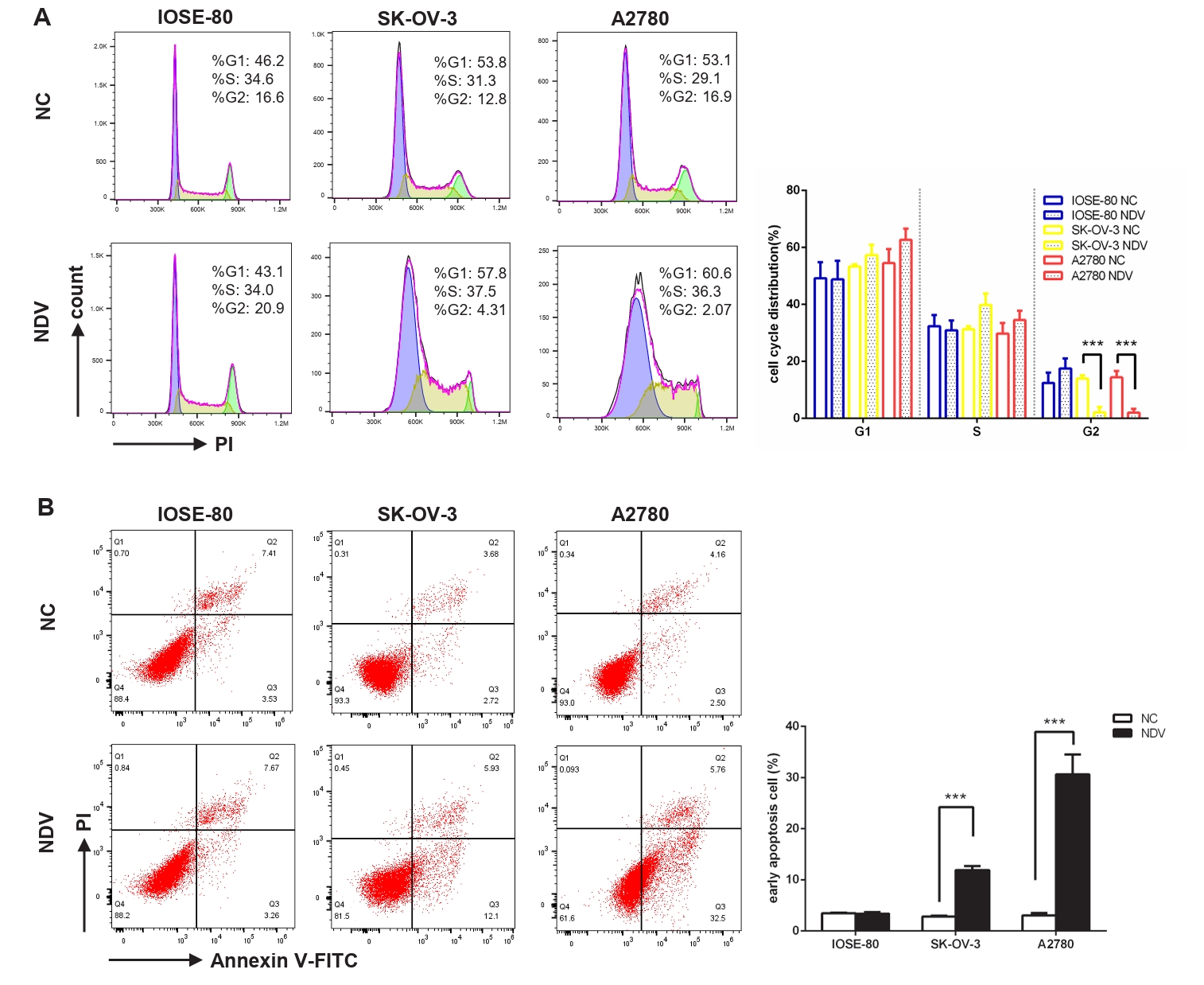

Fig. 3.The migration and invasion of ovarian cancer cells were inhibited by infection with NDV. (A) Wound healing assay was carried out to detect the cell migration. Transwell assay was performed. The matrix gel uncoated inserts were used for migration assay (B) and matrix gel coated inserts were used for invasion assay (C) (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
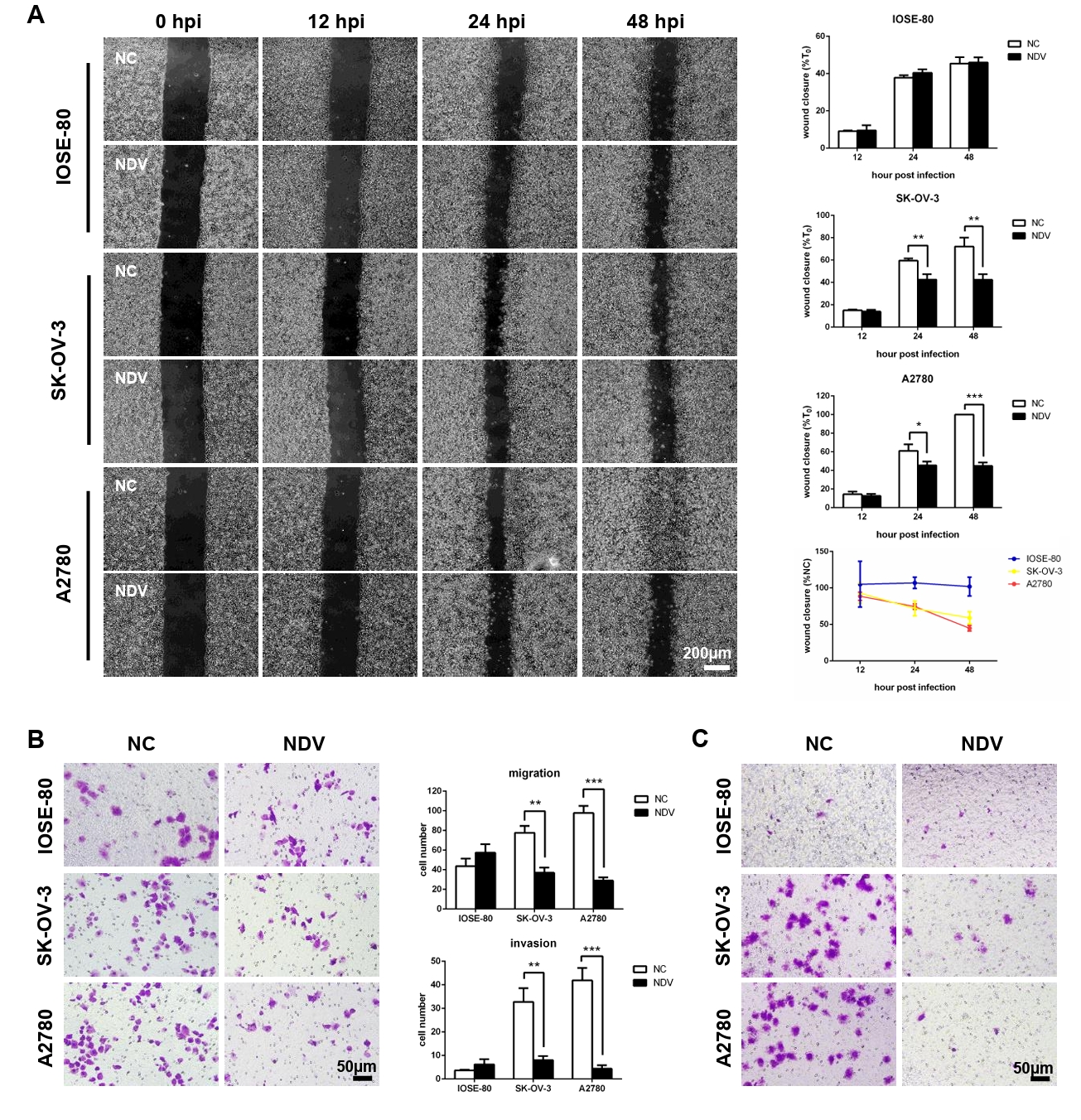

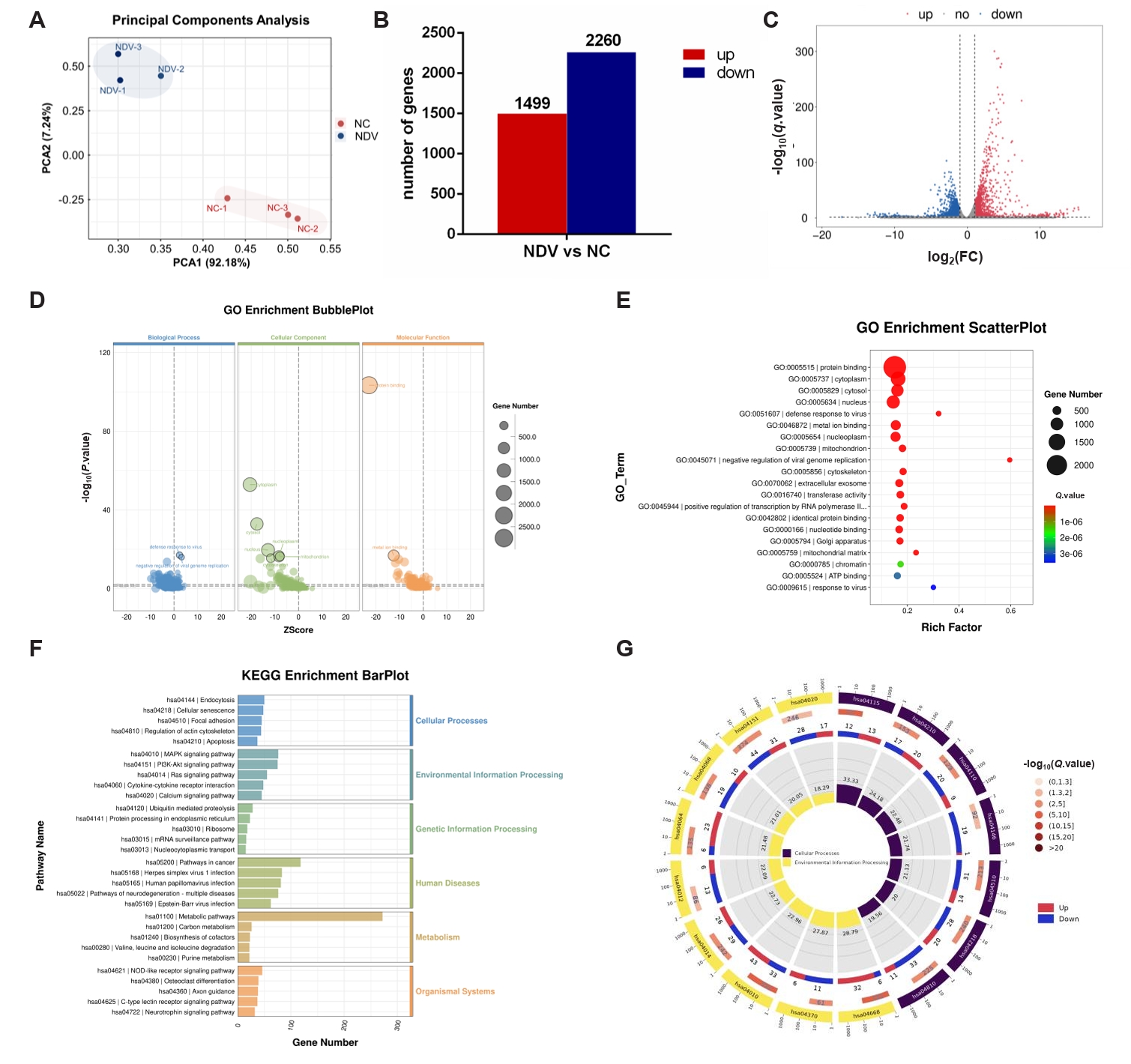
Fig. 4.DEGs in ovarian cancer cells A2780 between the NC and NDV-infected groups. (A) Correlation among samples in the NC and NDV groups. (B) Number of significantly up-regulated and down-regulated genes in different groups. (C) Volcano plot of identified genes including up-regulated and down-regulated genes in the RNA-seq. (D) GO enrichment bubbleplot of DEGs. (E) The top 20 enriched significantly differential GO terms of DEGs. (F) KEGG enrichment barplot of DEGs. (G) The significantly enriched KEGG pathways related to the KEGG_Level_1 cellular processes and environment information processing.
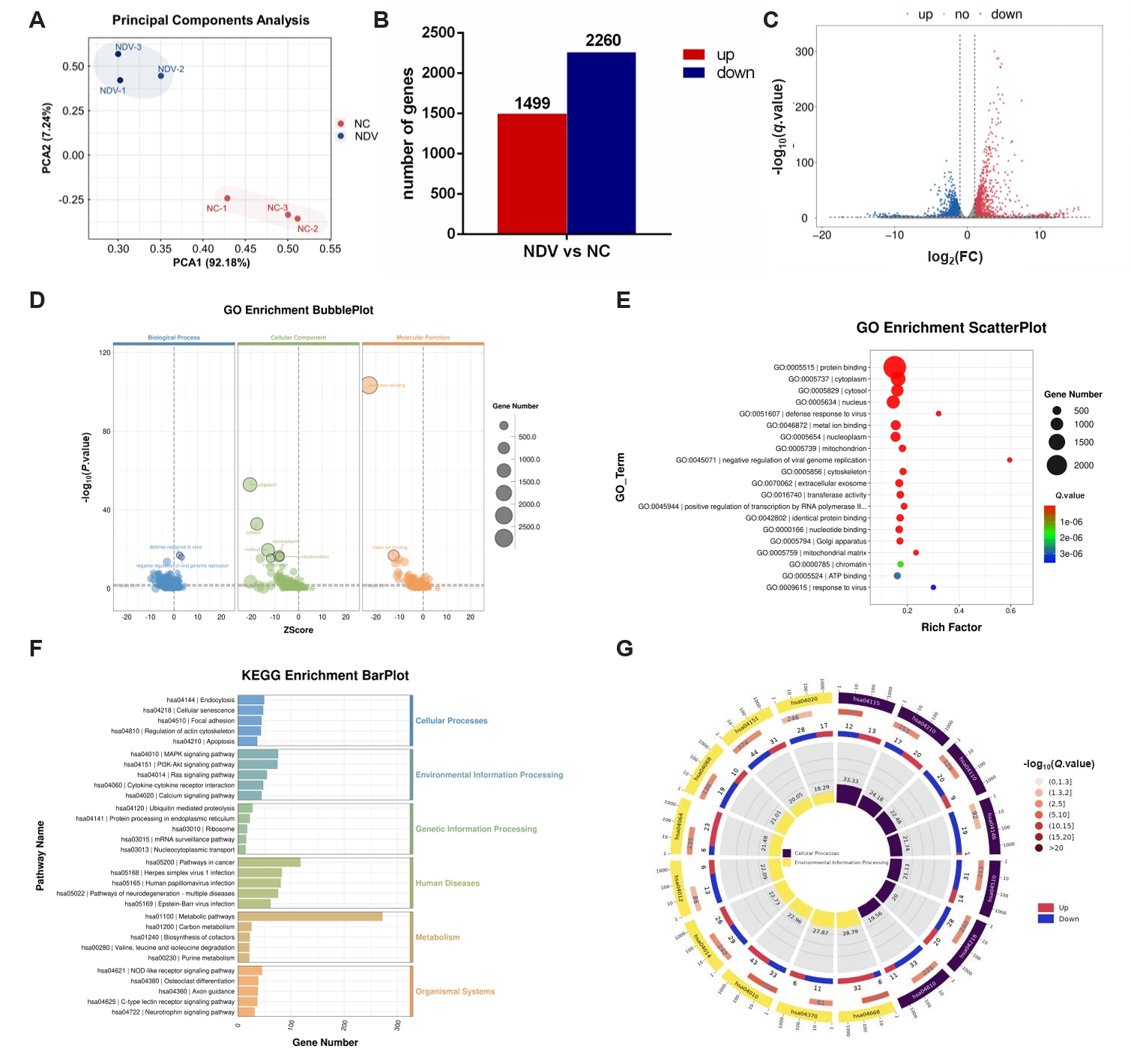

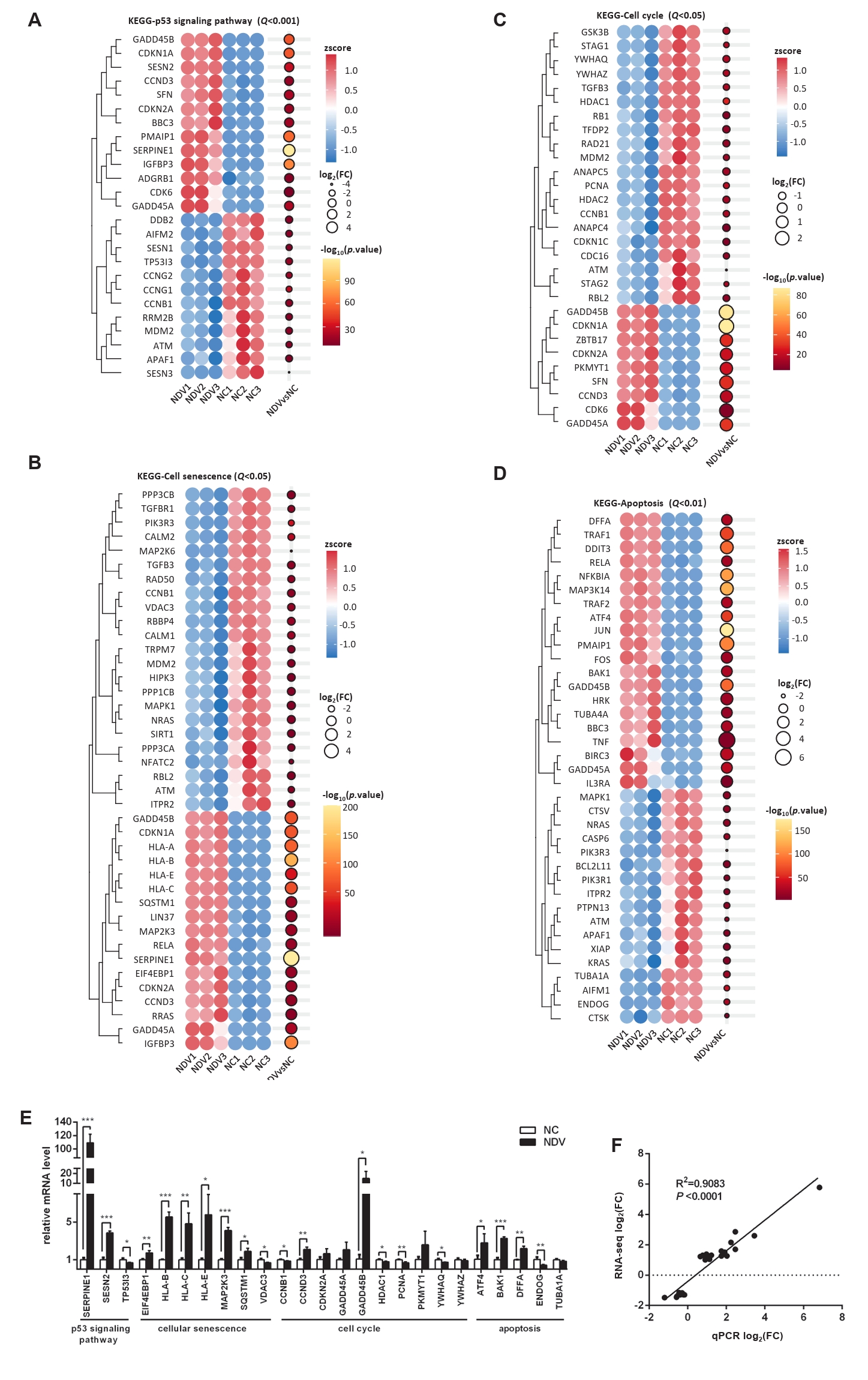
Fig. 5.Effect of NDV infection on the pathways related to cell growth and death. DEGs in the pathways p53 signaling pathway (A), cellular senescence (B), cell cycle (C), and apoptosis (D). When the number of DEGs enriched in the pathway is over 40, the top 40 significantly differential DEGs are displayed. (E) The mRNA expression levels of the screened DEGs were detected by qPCR. (F) The correlation between results from qPCR and RNA-seq (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
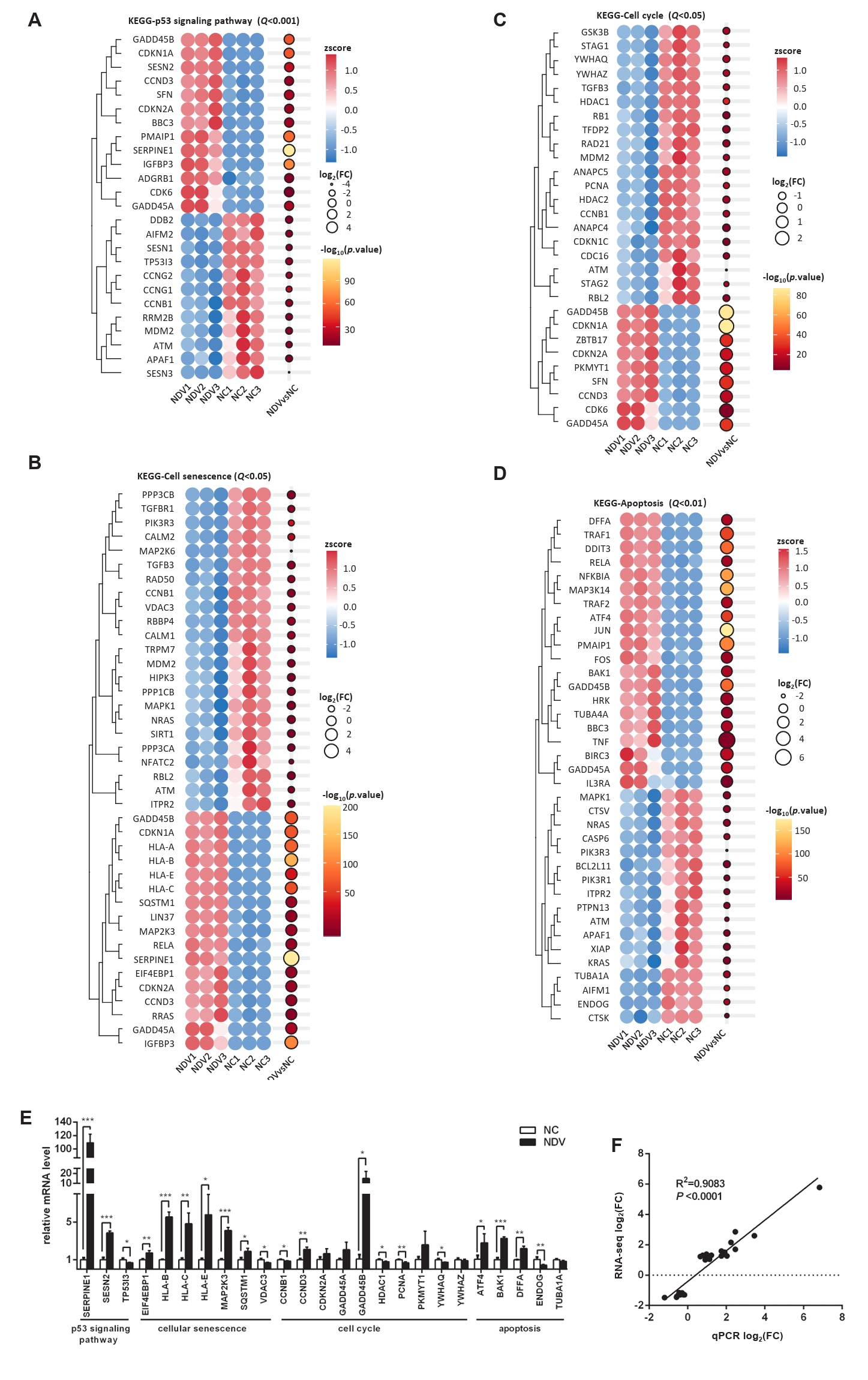

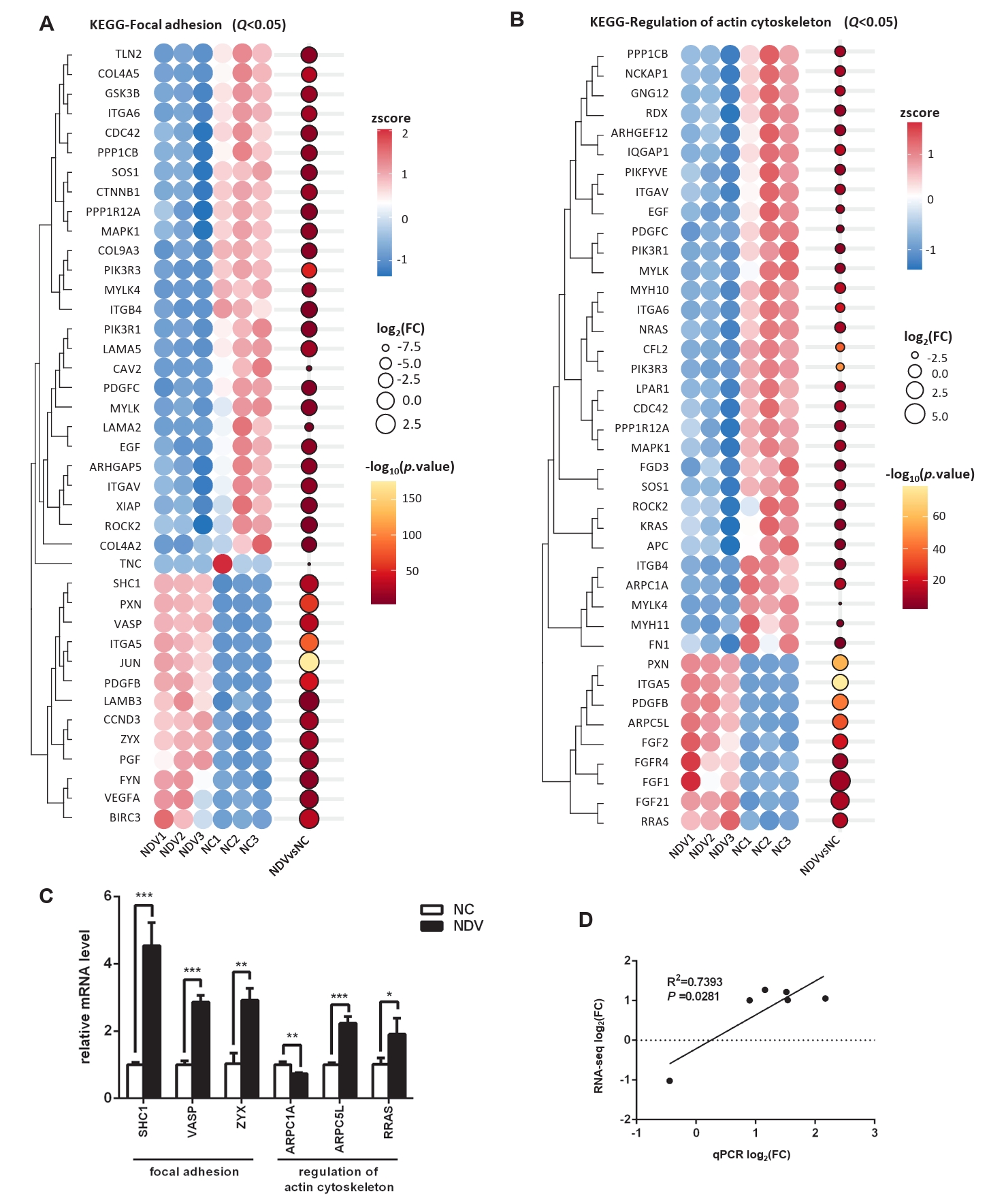
Fig. 6.Effect of NDV infection on the pathways related to cell migration and invasion. DEGs in the pathways focal adhesion (A) and regulation of actin cytoskeleton (B). When the number of DEGs enriched in the pathway is over 40, the top 40 significantly differential DEGs are displayed. (C) The mRNA expression levels of the screened DEGs were detected by qPCR. (D) The correlation between results from qPCR and RNA-seq (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).

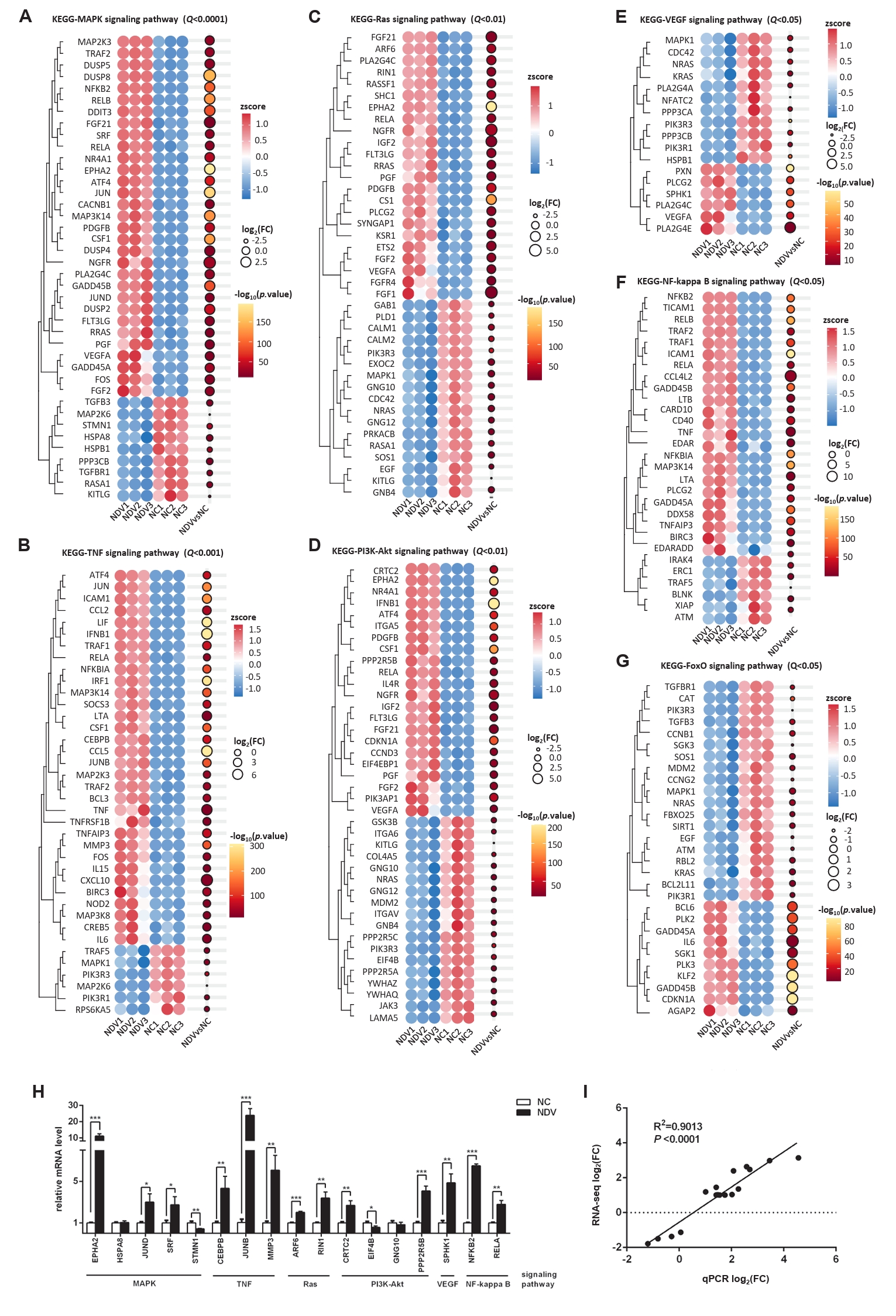
Fig. 7.Effect of NDV infection on the pathways related to signal transduction. DEGs in the pathways MAPK signaling pathway (A), TNF signaling pathway (B), Ras signaling pathway (C), PI3K-Akt signaling pathway (D), VEGF signaling pathway (E), NF-kappa B signaling pathway (F), and FoxO signaling pathway (G). When the number of DEGs enriched in the pathway is over 40, the top 40 significantly differential DEGs are displayed. (H) The mRNA expression levels of the screened DEGs were detected by qPCR. (I) The correlation between results from qPCR and RNA-seq (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
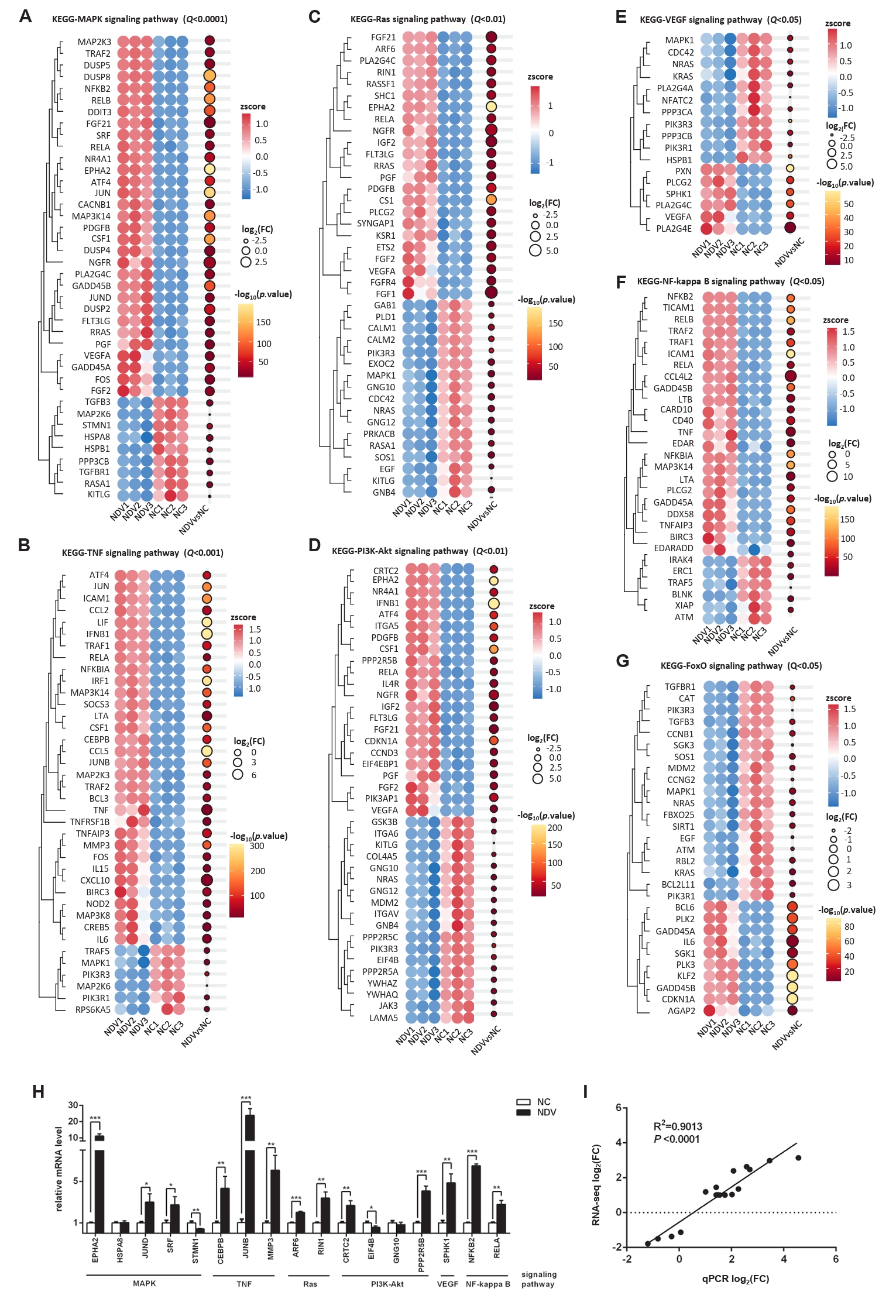

Fig. 8.The interaction among pathways and DEGs. (A) The gene-pathway network showed the connection among pathways via some DEGs. (B) The PPI network indicated the interaction among DEGs, helping us find the relatively important DEGs.
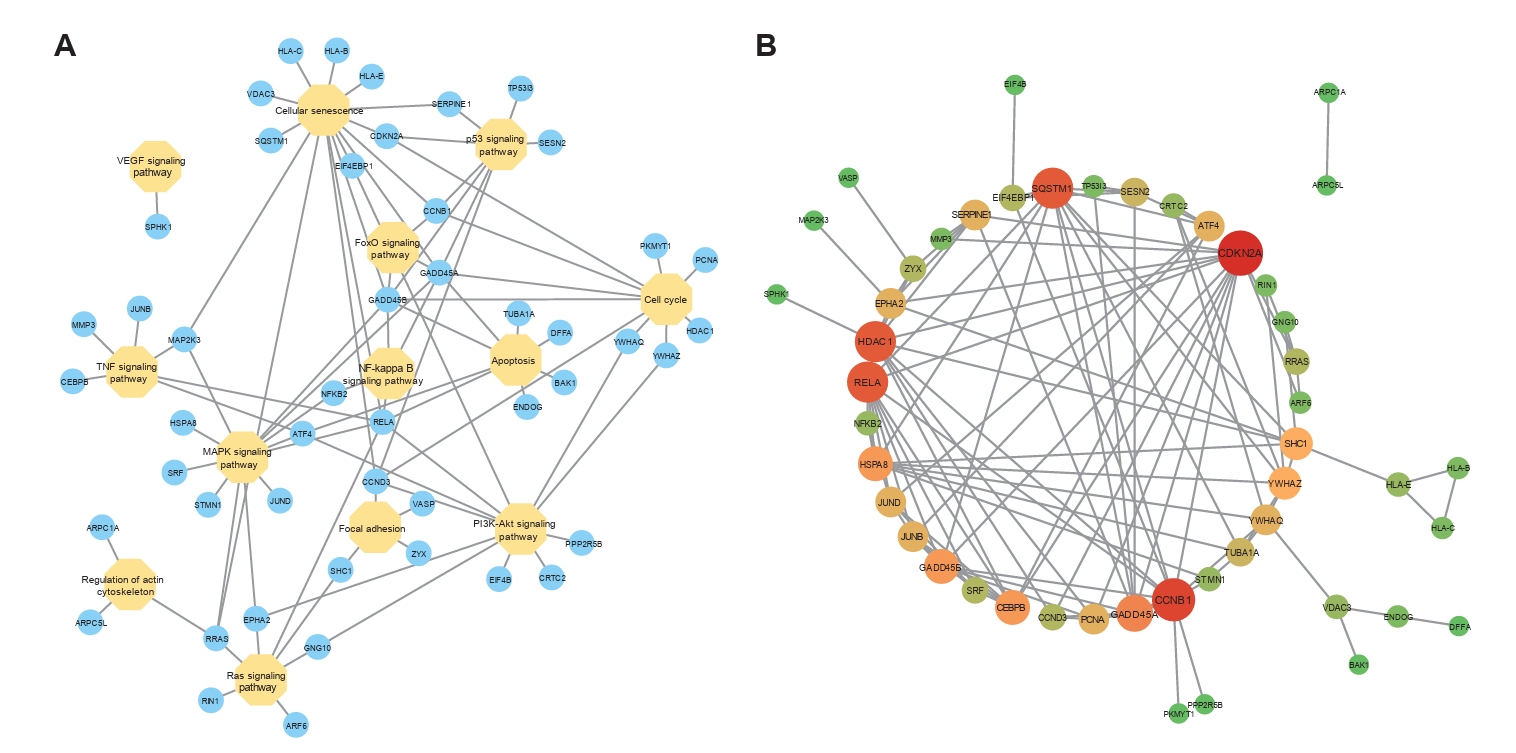

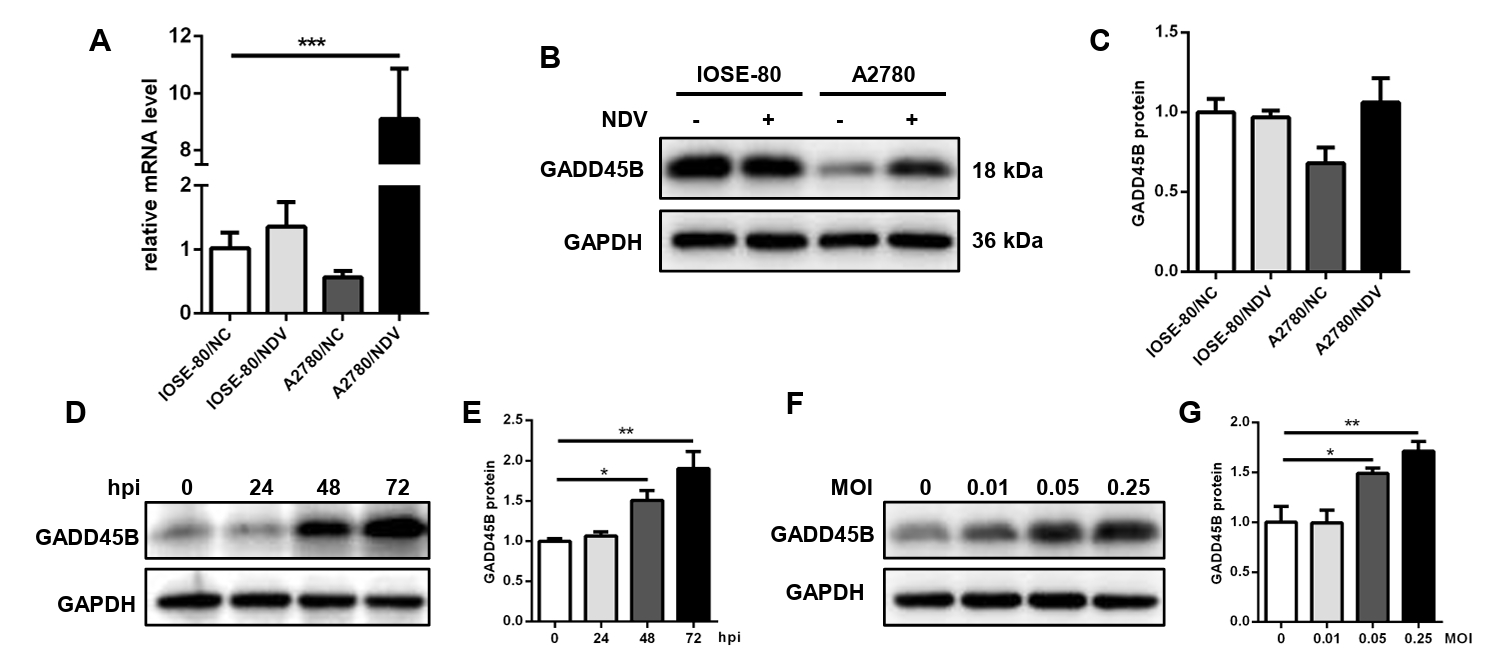
Fig. 9.The expression level of GADD45B in cells A2780 and IOSE-80. The mRNA expression level (A) and protein expression level (B, C) of GADD45B in A2780 and IOSE-80. The protein expression of GADD45B in A2780 showed a positive correlation with hpi (D, E) and MOI of NDV infection (F, G) (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
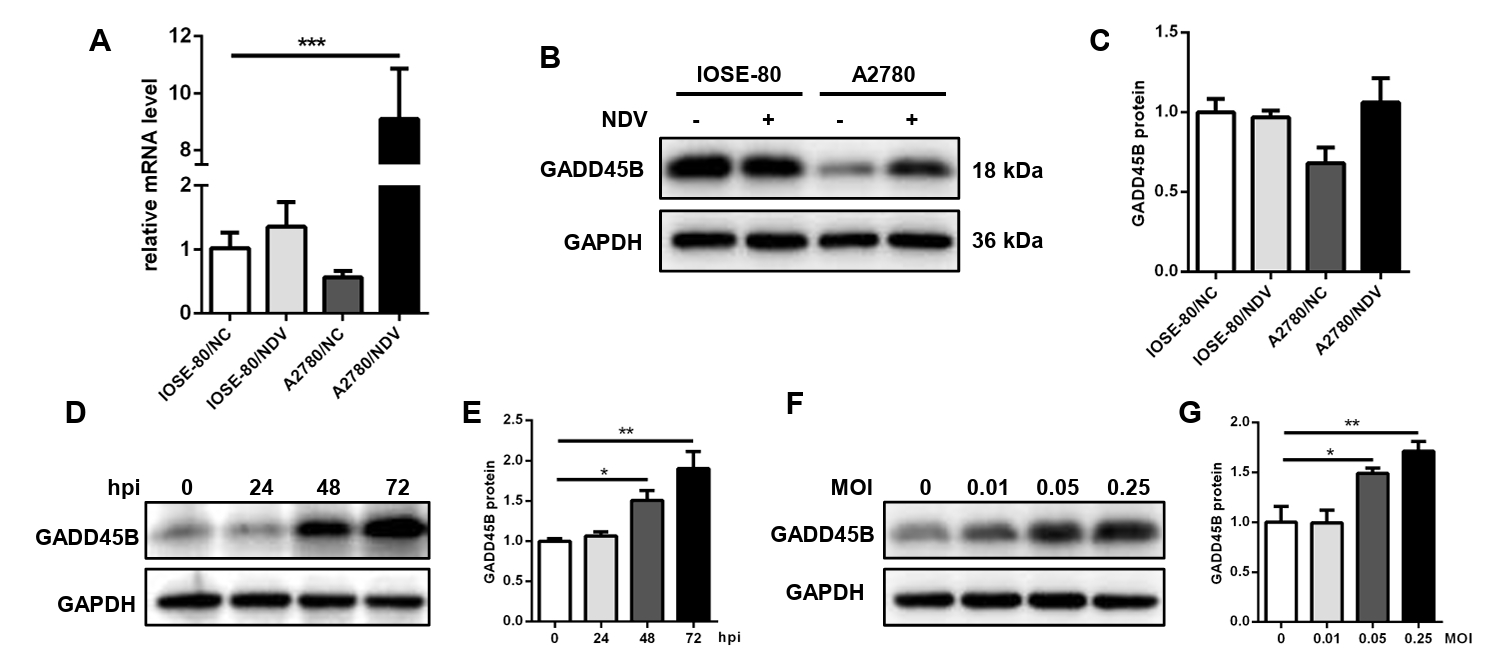

Table 1.Thirteen important pathways involved in NDV-induced phenotypical changes in ovarian cancer cells
- Ahlert T, Sauerbrei W, Bastert G, Ruhland S, Bartik B, et al. 1997. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J Clin Oncol. 15(4): 1354–1366. ArticlePubMed
- Bohle W, Schlag P, Liebrich W, Hohenberger P, Manasterski M, et al. 1990. Postoperative active specific immunization in colorectal cancer patients with virus-modified autologous tumor-cell vaccine. First clinical results with tumor-cell vaccines modified with live but avirulent Newcastle disease virus. Cancer. 66(7): 1517–1523. ArticlePubMed
- Bu Y, Li X, He Y, Huang C, Shen Y, et al. 2016. A phosphomimetic mutant of RelA/p65 at Ser536 induces apoptosis and senescence: An implication for tumor-suppressive role of Ser536 phosphorylation. Int J Cancer. 138(5): 1186–1198. ArticlePubMed
- Buijs PR, van Amerongen G, vanNieuwkoop S, Bestebroer TM, van Run PR, et al. 2014. Intravenously injected Newcastle disease virus in non-human primates is safe to use for oncolytic virotherapy. Cancer Gene Ther. 21(11): 463–471. ArticlePubMedPDF
- Cassel WA, Garrett RE. 1965. Newcastle disease virus as an antineoplastic agent. Cancer. 18: 863–868. ArticlePubMed
- Chen L, Fang C, Yuan X, Liu M, Wu P, et al. 2023. Has-miR-300-GADD45B promotes melanoma growth via cell cycle. Aging (Albany NY). 15(23): 13920–13943. ArticlePubMedPMC
- Deneka AY, Baca Y, Serebriiskii IG, Nicolas E, Parker MI, et al. 2022. Association of TP53 and CDKN2A mutation profile with tumor mutation burden in head and neck cancer. Clin Cancer Res. 28(9): 1925–1937. ArticlePubMedPMCPDF
- Dittmann M, Hoffmann HH, Scull MA, Gilmore RH, Bell KL, et al. 2015. A serpin shapes the extracellular environment to prevent influenza A virus maturation. Cell. 160(4): 631–643. ArticlePubMedPMC
- Elankumaran S, Rockemann D, Samal SK. 2006. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J Virol. 80(15): 7522–7534. ArticlePubMedPMCLink
- Feng H, Wei D, Nan G, Cui SJ, Chen ZN, et al. 2011. Construction of a minigenome rescue system for Newcastle disease virus strain Italien. Arch Virol. 156(4): 611–616. ArticlePubMedPDF
- Gal-Ben-Ari S, Barrera I, Ehrlich M, Rosenblum K. 2018. PKR: A kinase to remember. Front Mol Neurosci. 11: 480.ArticlePubMedPMC
- Geismann C, Hauser C, Grohmann F, Schneeweis C, Bölter N, et al. 2023. NF-κB/RelA controlled A20 limits TRAIL-induced apoptosis in pancreatic cancer. Cell Death Dis. 14: 3.ArticlePubMedPMCPDF
- Karcher J, Dyckhoff G, Beckhove P, Reisser C, Brysch M, et al. 2004. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res. 64(21): 8057–8061. ArticlePubMedPDF
- Kim T, Ko SG. 2021. JI017, a complex herbal medication, induces apoptosis via the NOX4-PERK-CHOP axis in ovarian cancer cells. Int J Mol Sci. 22(22): 12264.Article
- Kirchner HH, Anton P, Atzpodien J. 1995. Adjuvant treatment of locally advanced renal cancer with autologous virus-modified tumor vaccines. World J Urol. 13(3): 171–173. ArticlePubMedPDF
- Konstantinopoulos PA, Matulonis UA. 2023. Clinical and translational advances in ovarian cancer therapy. Nat Cancer. 4: 1239–1257. ArticlePubMedPDF
- Li Y, Shen L, Tao K, Xu G, Ji K. 2023. Key roles of p53 signaling pathway-related factors GADD45B and SERPINE1 in the occurrence and development of gastric cancer. Mediators Inflamm. 2023: 6368893.ArticlePubMedPMCPDF
- Liang Y, Tian WY, Huang JJ, Gao LX, Fan XH. 2021. MicroRNA-204 plays a role as a tumor suppressor in Newcastle disease virus-induced oncolysis in lung cancer A549 cells. Oncol Lett. 21(6): 482.ArticlePubMedPMC
- Liu Y, Liu Y, Huang Y, Wen H, Zhao L, et al. 2021. The effect of the HRB linker of Newcastle disease virus fusion protein on the fusogenic activity. J Microbiol. 59(5): 513–521. ArticlePubMedPDF
- Liu Y, Su Z, Tavana O, Gu W. 2024. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell. 42(6): 946–967. ArticlePubMedPMC
- Liu Y, Sun C, Chi M, Wen H, Zhao L, et al. 2019. Genetic characterization and phylogenetic analysis of Newcastle disease virus from China. Infect Genet Evol. 75: 103958.ArticlePubMed
- Lou J, Geng S, He W, Liu SB, Shi X, et al. 2020. Zyxin inhibits the epithelial-mesenchymal transition process in gastric cancer by upregulating SIRT1. MedComm. 4(5): e357.Article
- Manohar SM. 2024. At the crossroads of TNF-α signaling and cancer. Curr Mol Pharmacol. 17(1): e060923220758. ArticleLink
- Marin I, Boix O, Garcia-Garijo A, Sirois I, Caballe A, et al. 2023. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 13(2): 410–431. ArticlePubMedPDF
- Matuszewska K, Santry LA, van Vloten JP, AuYeung AWK, Major PP, et al. 2019. Combining vascular normalization with an oncolytic virus enhances immunotherapy in a preclinical model of advanced-stage ovarian cancer. Clin Cancer Res. 25(5): 1624–1638. ArticlePubMedPDF
- Murray DR, Cassel WA, Torbin AH, Olkowski ZL, Moore ME. 1977. Viral oncolysate in the management of malignant melanoma. II. Clinical studies. Cancer. 40(2): 680–686. ArticlePubMedLink
- Nebgen DR, Lu KH, Bast RC Jr. 2019. Novel approaches to ovarian cancer screening. Curr Oncol Rep. 21(8): 75.ArticlePubMedPMCPDF
- Okami H, Ozawa N, Sohda M, Yokobori T, Osone K, et al. 2023. HLA class I expression is associated with DNA damage and immune cell infiltration into dysplastic and neoplastic lesions in ulcerative colitis. Int J Mol Sci. 24(17): 13648.ArticlePubMedPMC
- Pan JX, Qu F, Wang FF, Xu J, Mu LS, et al. 2017. Aberrant SERPINE1 DNA methylation is involved in carboplatin-induced epithelial-mesenchymal transition in epithelial ovarian cancer. Arch Gynecol Obstet. 296(6): 1145–1152. ArticlePubMedPDF
- Pomer S, Schirrmacher V, Thiele R, Lohrke H, Brkovic D, et al. 1995. Tumor response and 4-year survival data of patients with advanced renal-cell carcinoma treated with autologous tumor vaccine and subcutaneous rIL-2 and IFN-α2b. Int J Oncol. 6(5): 947–954. ArticlePubMed
- Rangaswamy US, Wang W, Cheng X, McTamney P, Carroll D, et al. 2017. Newcastle disease virus establishes persistent infection in tumor cells in vitro: Contribution of the cleavage site of fusion protein and second sialic acid binding site of hemagglutinin-neuraminidase. J Virol. 91(16): e00770–17. ArticlePubMedPMCLink
- Reichard KW, Lorence RM, Cascino CJ, Peeples ME, Walter RJ, et al. 1992. Newcastle disease virus selectively kills human tumor cells. J Surg Res. 52(5): 448–453. ArticlePubMed
- Schirrmacher V. 2015. Oncolytic Newcastle disease virus as a prospective anti-cancer therapy. A biologic agent with potential to break therapy resistance. Expert Opin Biol Ther. 15(12): 1757–1771. ArticlePubMed
- Schirrmacher V. 2016. Fifty years of clinical application of Newcastle disease virus: Time to celebrate! Biomedicines. 4(3): 16.ArticlePubMedPMC
- Schirrmacher V. 2022. Molecular mechanisms of anti-neoplastic and immune stimulatory properties of oncolytic Newcastle disease virus. Biomedicines. 10(3): 562.ArticlePubMedPMC
- Shin J, Bae J, Park S, Kang HG, Shin SM, et al. 2020. mTOR-dependent role of Sestrin2 in regulating tumor progression of human endometrial cancer. Cancers (Basel). 12(9): 2515.ArticlePubMedPMC
- Siegel RL, Giaquinto AN, Jemal A. 2024. Cancer statistics, 2024. CA Cancer J Clin. 74(1): 12–49. ArticlePubMed
- Steiner HH, Bonsanto MM, Beckhove P, Brysch M, Geletneky K, et al. 2004. Antitumor vaccination of patients with glioblastoma multiforme: A pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol. 22(21): 4272–4281. ArticlePubMed
- Tang H, Kang R, Liu J, Tang D. 2024. ATF4 in cellular stress, ferroptosis, and cancer. Arch Toxicol. 98(4): 1025–1041. ArticlePubMedPDF
- Uchihara Y, Shibata A. 2023. Regulation of DNA damage-induced HLA class I presentation. DNA Repair (Amst). 132: 103590.ArticlePubMed
- Washburn B, Schirrmacher V. 2002. Human tumor cell infection by Newcastle disease virus leads to upregulation of HLA and cell adhesion molecules and to induction of interferons, chemokines and finally apoptosis. Int J Oncol. 21(1): 85–93. ArticlePubMed
- Webb PM, Jordan SJ. 2024. Global epidemiology of epithelial ovarian cancer. Nat Rev Clin Oncol. 21(5): 389–400. ArticlePubMedPDF
- Wei JL, Fang M, Fu ZX, Zhang SR, Guo JB, et al. 2017. Sestrin 2 suppresses cell proliferation through AMPK/mTORC1 pathway activation in colorectal cancer. Oncotarget. 8(30): 49318–49328. ArticlePubMedPMC
- Yaacov B, Eliahoo E, Lazar I, Ben-Shlomo M, Greenbaum I, et al. 2008. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 15(12): 795–807. ArticlePubMedPDF
- Yang JD, Ma L, Zhu Z. 2019. SERPINE1 as a cancer-promoting gene in gastric adenocarcinoma: Facilitates tumor cell proliferation, migration, and invasion by regulating EMT. J Chemother. 31(7-8): 408–418. ArticlePubMed
- Yang X, Tang S, Li D, Yu X, Wang F, et al. 2018. DIDS inhibits overexpression BAK1-induced mitochondrial apoptosis through GSK3β/β-catenin signaling pathway. J Cell Physiol. 233(6): 5070–5077. ArticlePubMedLink
- Yang C, Xia BR, Zhang ZC, Zhang YJ, Lou G, et al. 2020. Immunotherapy for ovarian cancer: Adjuvant, combination, and neoadjuvant. Front Immunol. 11: 577869.ArticlePubMedPMC
- Zhan F, Guo Y, He L. 2024. A novel defined programmed cell death-related gene signature for predicting the prognosis of serous ovarian cancer. J Ovarian Res. 17(1): 92.ArticlePubMedPMCPDF
- Zhang JH, Xu M. 2000. DNA fragmentation in apoptosis. Cell Res. 10: 205–211. ArticlePubMedPDF
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Newcastle Disease Virus Vaccine Strain H as a Potential Oncolytic Agent in Ovarian Cancer Therapy
V. A. Sarkisova, S. Sh. Karshieva, A. A Makarova, D. O. Neymysheva, P. M. Chumakov
Molecular Biology.2025; 59(6): 1055. CrossRef
The key pathways and genes related to oncolytic Newcastle disease virus-induced phenotypic changes in ovarian cancer cells
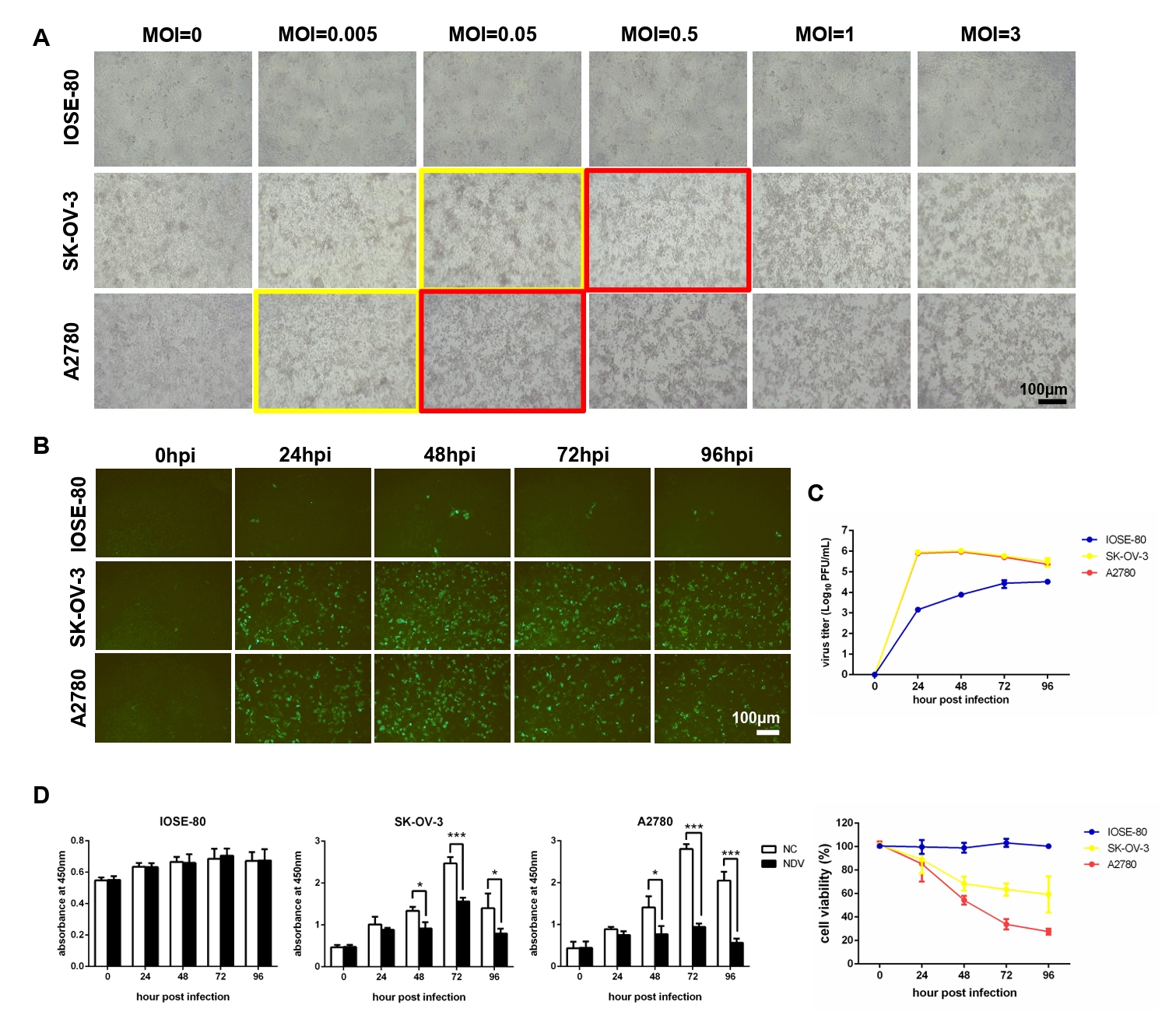
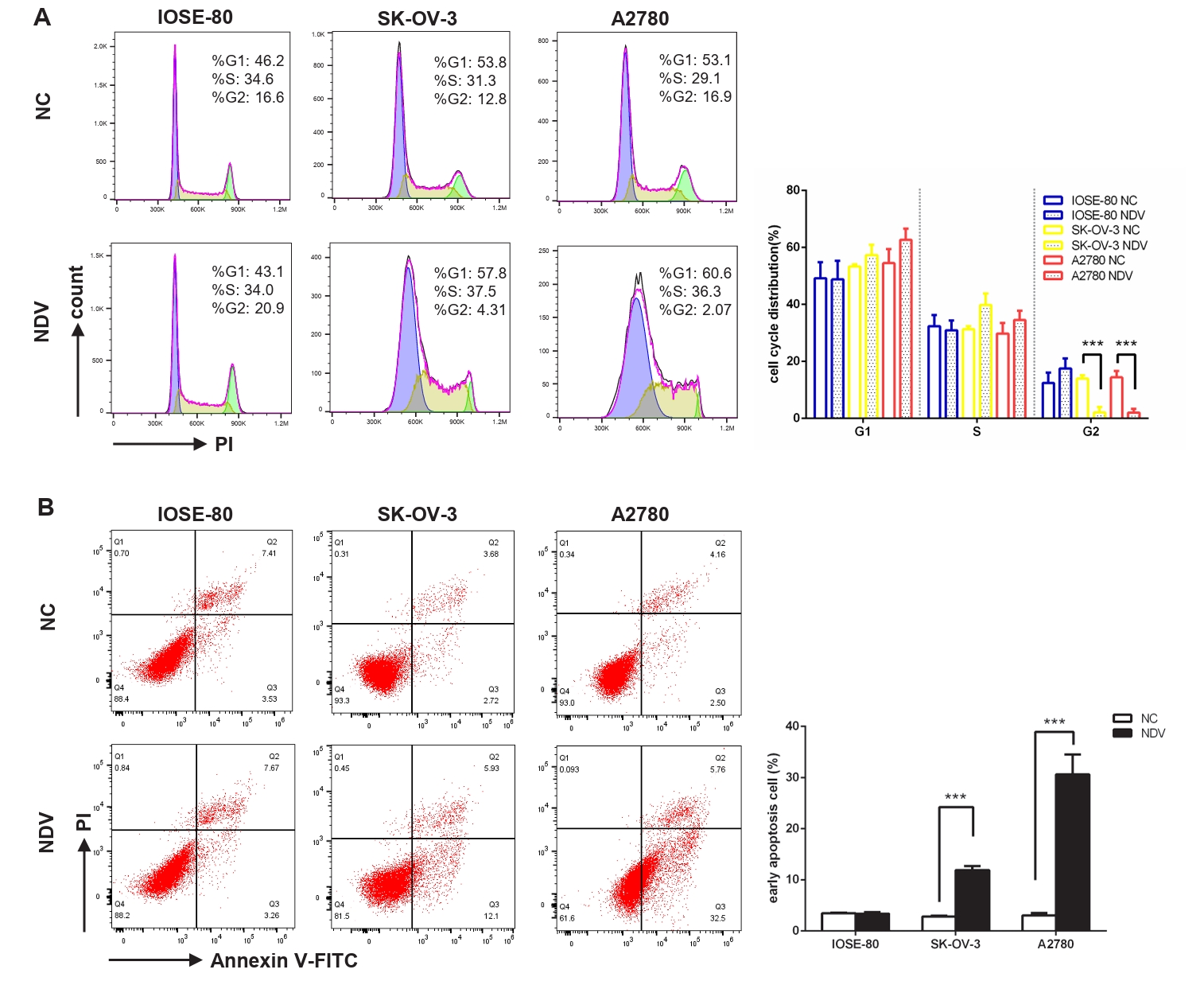
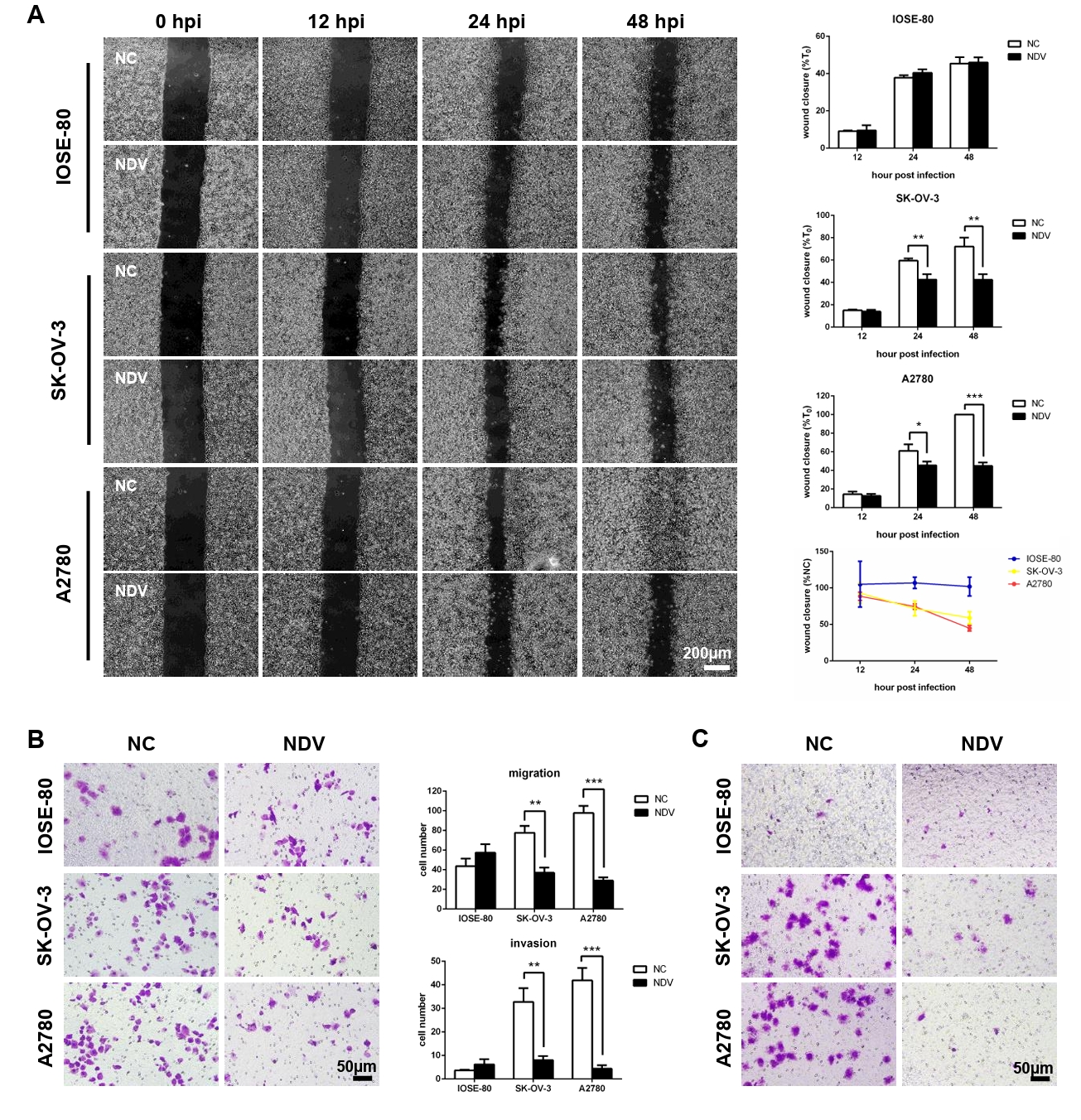
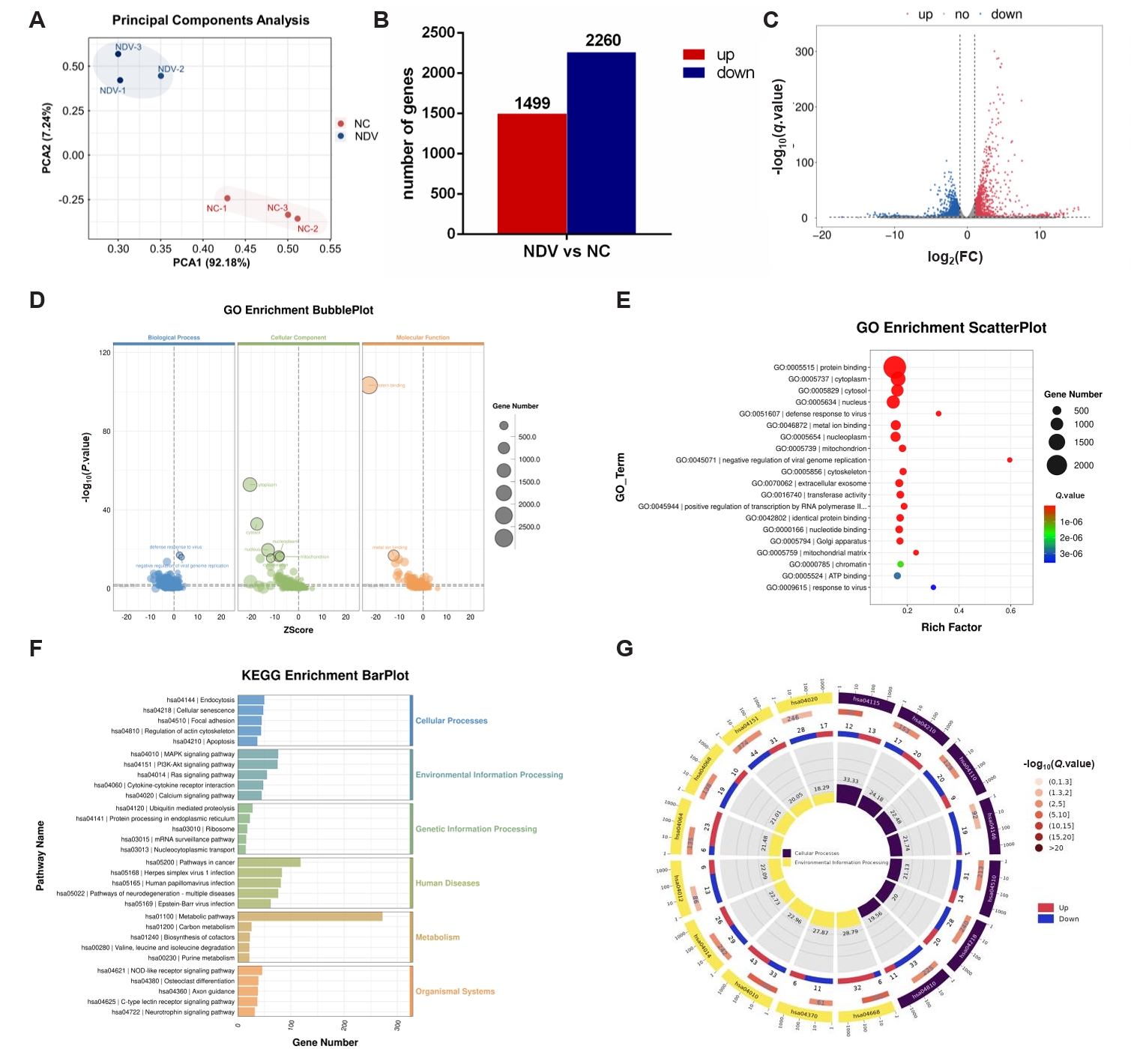
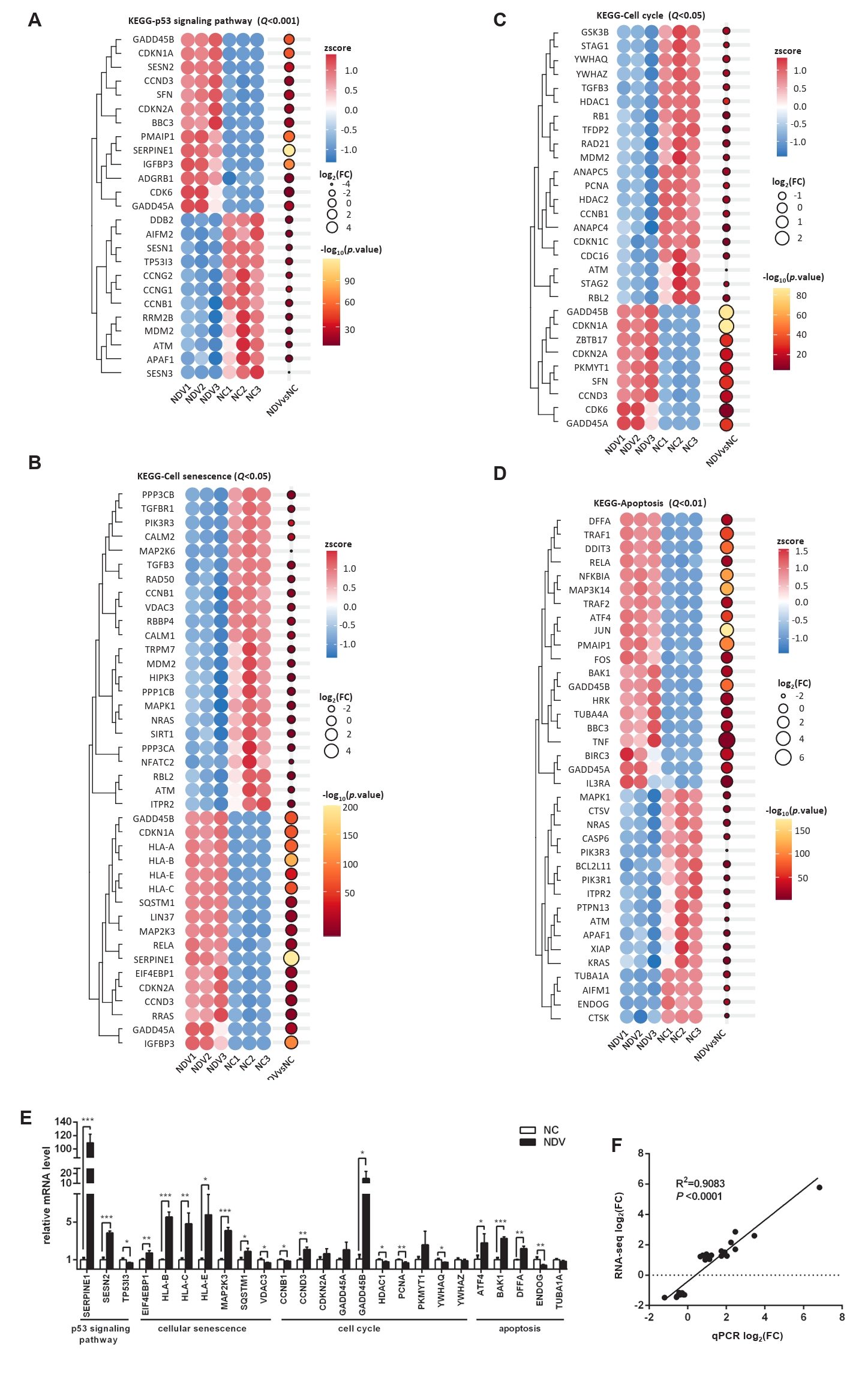
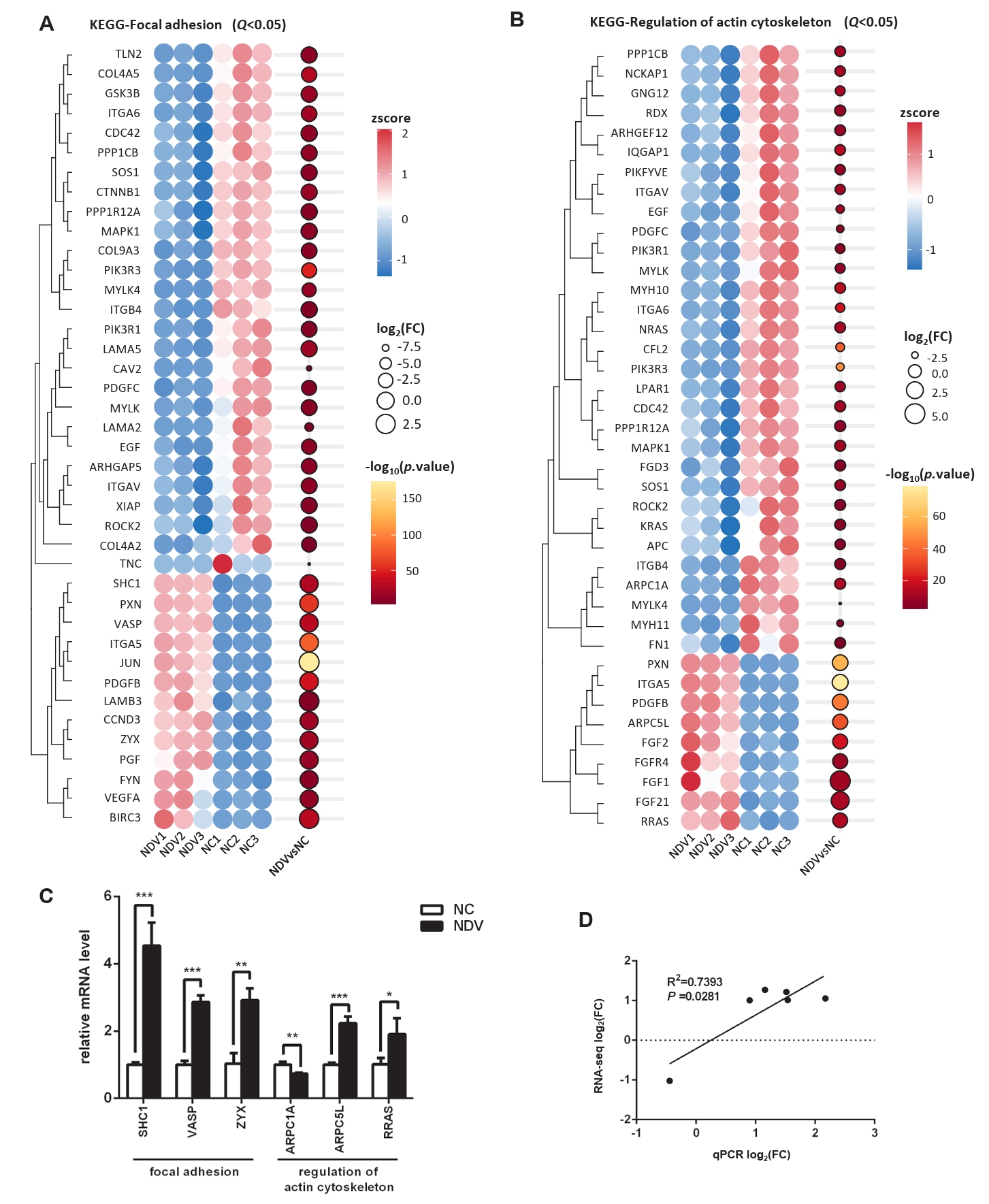
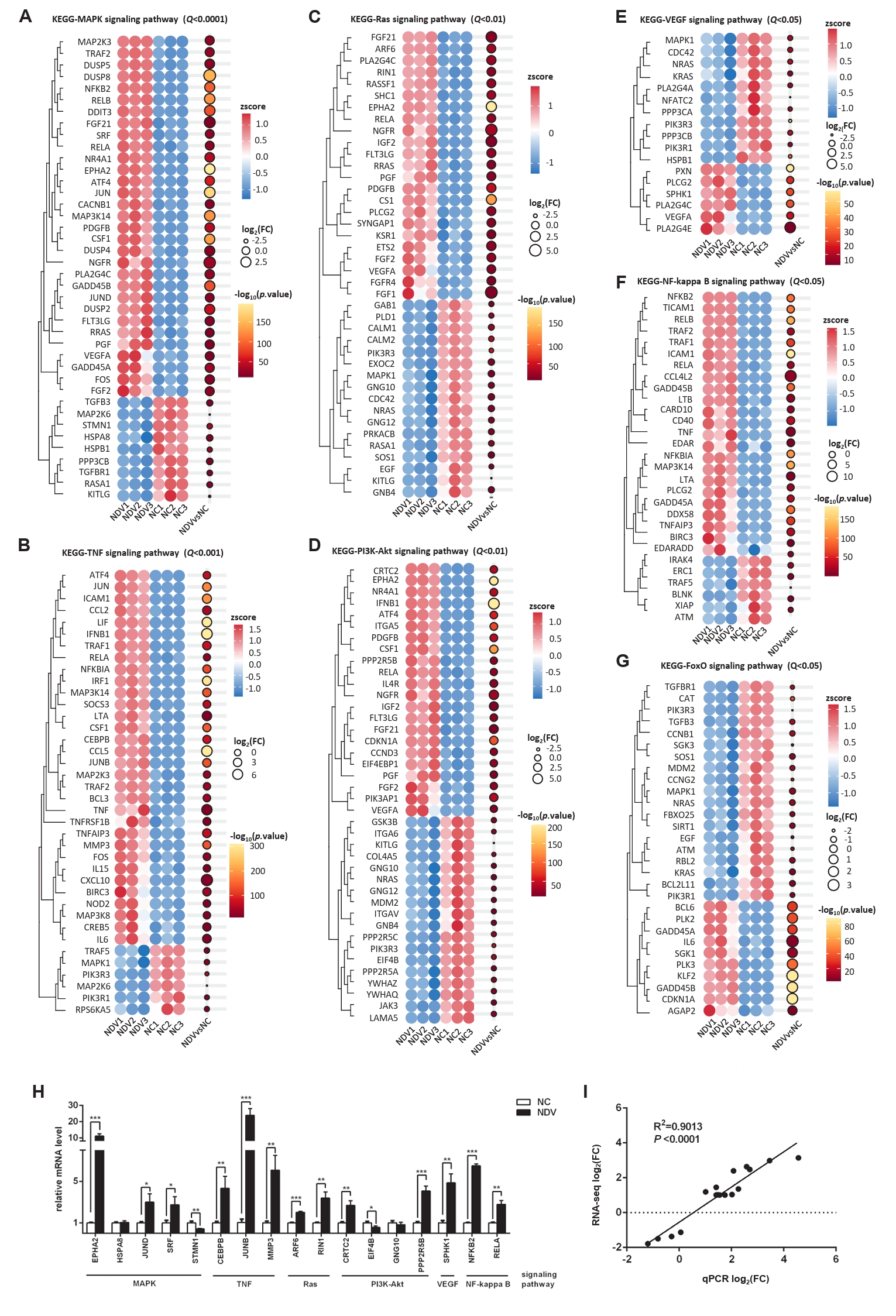
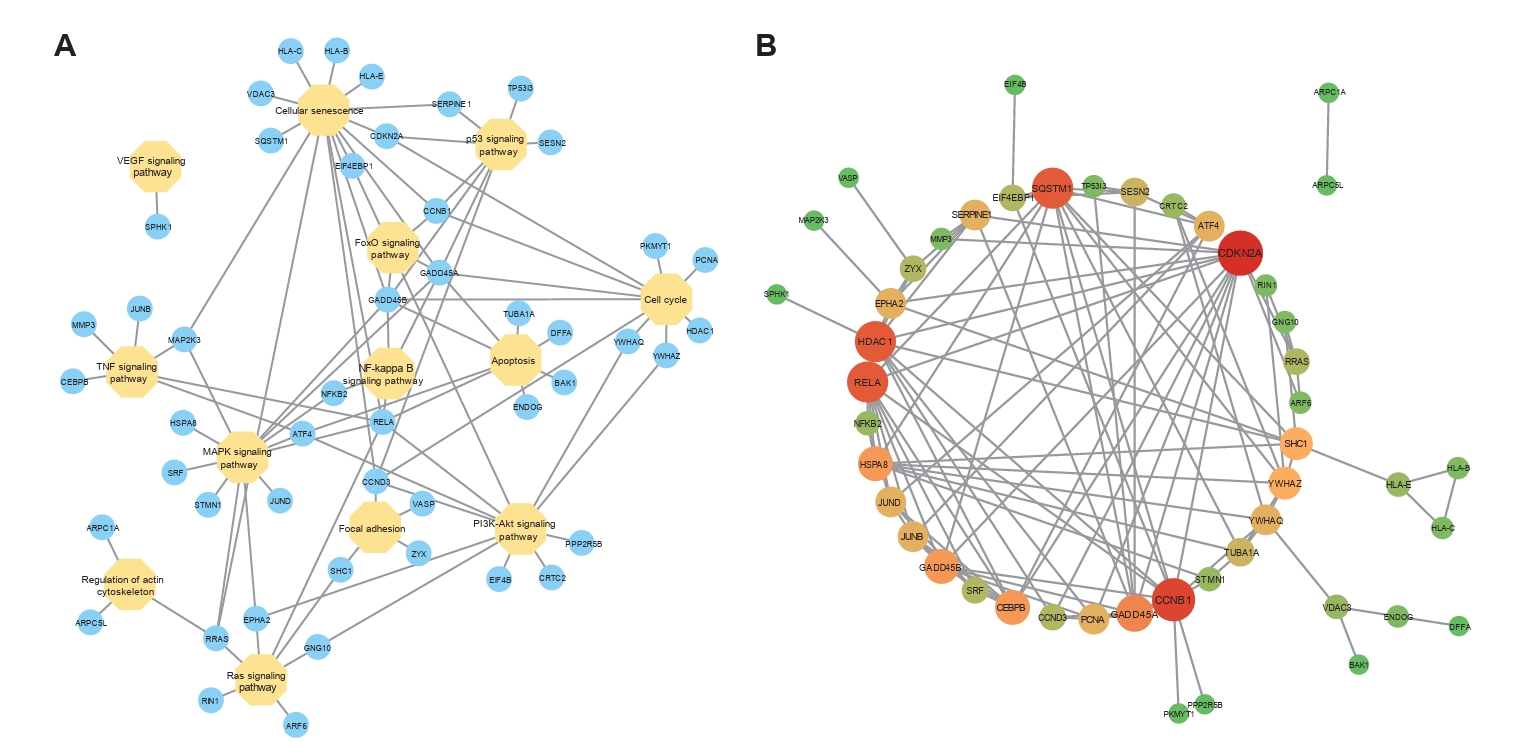
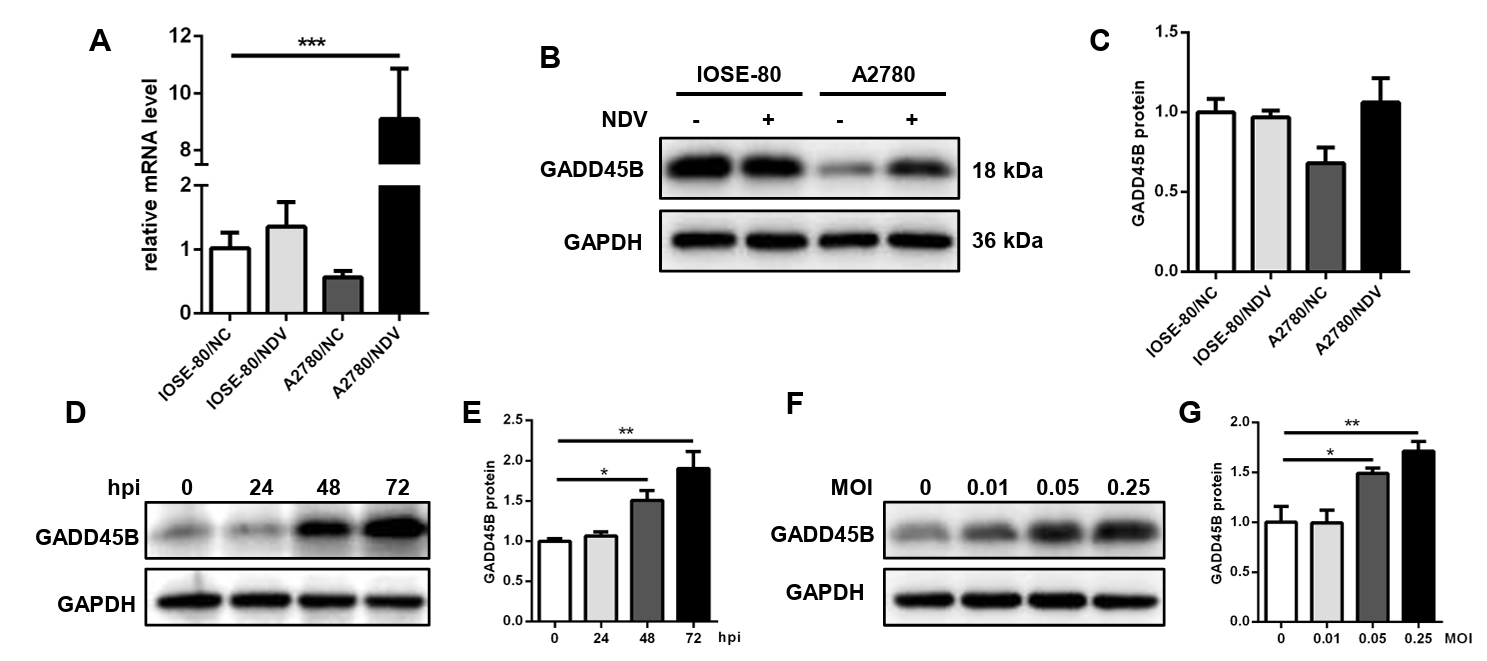
Fig. 1. NDV can grow speedily in ovarian cancer cells and suppress cell proliferation. (A) Determination of the optimal MOI of NDV by observing the CPE. The yellow rectangles indicate the onset of CPE and the red rectangles indicate the obvious CPE. The multi-step growth of NDV was detected by IFA (B) and plaque assay (C). (D) The cell proliferation was detected by CCK-8 assay (*, P < 0.05; ***, P < 0.001; otherwise, P > 0.05).
Fig. 2. Infection with NDV caused a disrupted cell cycle and prompted apoptosis of ovarian cancer cells. Flow cytometry was performed to explore the influence of NDV on the cell cycle (A) and apoptosis (B) of ovarian cancer cells. The flow cytometry on the left displays a representative experimental result, while the histogram on the right shows the average results of three experiments (***, P < 0.001; otherwise, P > 0.05).
Fig. 3. The migration and invasion of ovarian cancer cells were inhibited by infection with NDV. (A) Wound healing assay was carried out to detect the cell migration. Transwell assay was performed. The matrix gel uncoated inserts were used for migration assay (B) and matrix gel coated inserts were used for invasion assay (C) (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
Fig. 4. DEGs in ovarian cancer cells A2780 between the NC and NDV-infected groups. (A) Correlation among samples in the NC and NDV groups. (B) Number of significantly up-regulated and down-regulated genes in different groups. (C) Volcano plot of identified genes including up-regulated and down-regulated genes in the RNA-seq. (D) GO enrichment bubbleplot of DEGs. (E) The top 20 enriched significantly differential GO terms of DEGs. (F) KEGG enrichment barplot of DEGs. (G) The significantly enriched KEGG pathways related to the KEGG_Level_1 cellular processes and environment information processing.
Fig. 5. Effect of NDV infection on the pathways related to cell growth and death. DEGs in the pathways p53 signaling pathway (A), cellular senescence (B), cell cycle (C), and apoptosis (D). When the number of DEGs enriched in the pathway is over 40, the top 40 significantly differential DEGs are displayed. (E) The mRNA expression levels of the screened DEGs were detected by qPCR. (F) The correlation between results from qPCR and RNA-seq (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
Fig. 6. Effect of NDV infection on the pathways related to cell migration and invasion. DEGs in the pathways focal adhesion (A) and regulation of actin cytoskeleton (B). When the number of DEGs enriched in the pathway is over 40, the top 40 significantly differential DEGs are displayed. (C) The mRNA expression levels of the screened DEGs were detected by qPCR. (D) The correlation between results from qPCR and RNA-seq (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
Fig. 7. Effect of NDV infection on the pathways related to signal transduction. DEGs in the pathways MAPK signaling pathway (A), TNF signaling pathway (B), Ras signaling pathway (C), PI3K-Akt signaling pathway (D), VEGF signaling pathway (E), NF-kappa B signaling pathway (F), and FoxO signaling pathway (G). When the number of DEGs enriched in the pathway is over 40, the top 40 significantly differential DEGs are displayed. (H) The mRNA expression levels of the screened DEGs were detected by qPCR. (I) The correlation between results from qPCR and RNA-seq (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
Fig. 8. The interaction among pathways and DEGs. (A) The gene-pathway network showed the connection among pathways via some DEGs. (B) The PPI network indicated the interaction among DEGs, helping us find the relatively important DEGs.
Fig. 9. The expression level of GADD45B in cells A2780 and IOSE-80. The mRNA expression level (A) and protein expression level (B, C) of GADD45B in A2780 and IOSE-80. The protein expression of GADD45B in A2780 showed a positive correlation with hpi (D, E) and MOI of NDV infection (F, G) (*, P < 0.05; **, P < 0.01; ***, P < 0.001; otherwise, P > 0.05).
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Fig. 8.
Fig. 9.
The key pathways and genes related to oncolytic Newcastle disease virus-induced phenotypic changes in ovarian cancer cells
| Pathway_ID | KEGG_Level_2 | Pathway_Name | Rich. Factor | S_Up_Num | S_Down_Num | P.value | Q.value |
|---|---|---|---|---|---|---|---|
| hsa04010 | Signal transduction | MAPK signaling pathway | 0.2296 | 43 | 33 | 1.21967E-06 | 8.0742E-05 |
| hsa04668 | Signal transduction | TNF signaling pathway | 0.2879 | 32 | 6 | 2.6516E-06 | 0.000146280 |
| hsa04115 | Cell growth and death | p53 signaling pathway | 0.3333 | 13 | 12 | 8.36252E-06 | 0.000395428 |
| hsa04014 | Signal transduction | Ras signaling pathway | 0.2273 | 26 | 29 | 4.88055E-05 | 0.001615463 |
| hsa04210 | Cell growth and death | Apoptosis | 0.2418 | 20 | 17 | 0.000222989 | 0.003354967 |
| hsa04151 | Signal transduction | PI3K-Akt signaling pathway | 0.2005 | 31 | 44 | 0.000198133 | 0.003354967 |
| hsa04510 | Cellular community - eukaryotes | Focal adhesion | 0.2113 | 14 | 31 | 0.001213301 | 0.013386752 |
| hsa04370 | Signal transduction | VEGF signaling pathway | 0.2787 | 6 | 11 | 0.002229870 | 0.019138009 |
| hsa04218 | Cell growth and death | Cellular senescence | 0.2000 | 20 | 28 | 0.002810453 | 0.022994128 |
| hsa04110 | Cell growth and death | Cell cycle | 0.2248 | 9 | 20 | 0.003335481 | 0.024048231 |
| hsa04810 | Cell motility | Regulation of actin cytoskeleton | 0.1956 | 11 | 33 | 0.006280651 | 0.037798102 |
| hsa04064 | Signal transduction | NF-kappa B signaling pathway | 0.2148 | 23 | 6 | 0.006583374 | 0.038912446 |
| hsa04068 | Signal transduction | FoxO signaling pathway | 0.2101 | 10 | 19 | 0.009004390 | 0.048269160 |
Table 1. Thirteen important pathways involved in NDV-induced phenotypical changes in ovarian cancer cells
Table 1.
TOP
 MSK
MSK

 ePub Link
ePub Link Cite this Article
Cite this Article